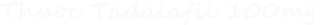7 Types of Erectile Dysfunction Treatments
What if a patient has taken a PDE5I, receives a nitrate, and becomes hypotensive from pronounced vasodilation? The American College of Cardiology and AHA suggest placing the patient in the Trendelenburg position, aggressive fluid resuscitation, and if necessary an α-agonist (phenylephrine), a β-agonist (norepinephrine), and intraaortic balloon counterpulsation. 23 There is no antidote to PDE5Is.
Tadalafil in the treatment of erectile dysfunction
The treatment for erectile dysfunction (ED) was revolutionized with the development of phosphodiesterase type 5 (PDE5) inhibitors. Tadalafil (Cialis ® ; Eli Lilly and Company, Indianapolis, IN, USA) is the newest and most versatile PDE5 inhibitor in the clinical armamentarium for the treatment of ED. Its most unique characteristic is its long half-life of 17.5 hours, which lends itself to a longer therapeutic window with on-demand dosing and effective steady-state plasma concentrations with once-daily dosing. Clinical trials have proven its safety and efficacy with both dosing strategies for all severities and etiologies of ED, including difficult-to-treat ED. This thorough review will discuss ED, the physiology of penile erection and the role of PDE5, and all aspects of tadalafil, from its development, through its pharmacology, to its latest clinical studies and indications.
Keywords: tadalafil, Cialis, PDE5 inhibitors, phosphodiesterase type 5, erectile dysfunction, penile erection
Introduction
Attempts at determining the neurotransmitter or neurotransmitters involved in creating an erection may some day lead to successful nonhormonal medical therapy [for erectile dysfunction] (Krane 1986, p. 731).
The last two decades have seen a dramatic growth of understanding in the physiology of erection, the pathophysiology of erectile dysfunction (ED), and its treatment options. As the above quote from the fifth edition of Campbell’s Urology reveals, it was not long ago when little was known of erectile physiology, and adequate treatment was still “some day” away. Prior to the turn of the century, the pharmacologic treatment options for ED were cumbersome and invasive, limited to intracavernosal injections or intraurethral pellets. Everything changed when oral phosphodiesterase type 5 (PDE5) inhibitors became available, completely revolutionizing the treatment of ED of all severities and etiologies. Pfizer introduced the first PDE5 inhibitor, sildenafil (Viagra ® ; Pfizer, New York, NY, USA), in March of 1998, and over the past 10 years, the new oral pharmacologic therapy together with an unbridled acceptance and newfound candor by the general public has not only dramatically increased the awareness and prevalence of ED, but also made treatment of the disease simple and effective. Once one of the most frustrating and refractory diseases for the urologic specialist, ED is now enthusiastically discussed and treated in the primary care setting.
The addition of two more PDE5 inhibitors to the market in 2003 broadened the landscape of ED treatment and solidified PDE5 inhibitors as safe and effective first-line treatments for ED. With the development of the new PDE5 inhibitors, attention was focused on increased potency as well as duration of action. Tadalafil (Cialis ® ; Eli Lilly and Company, Indianapolis, IN, USA), the newest of the three available PDE5 inhibitors, is similar to sildenafil and vardenafil (Levitra ® ; Bayer AG, Germany) in its mechanism of action, but differs primarily in its longer duration of action. In January 2008, Eli Lilly announced the latest innovation in the treatment of ED, Federal Drug Administration (FDA) approval for tadalafil once-daily dosing in the treatment of ED.
In this review, we will highlight ED, the physiology of penile erection, and the role of PDE5 before focusing on the newest and most versatile PDE5 inhibitor. We intend to thoroughly report tadalafil’s pharmacology, safety, and drug interactions; its efficacy, indications, and clinical trials; and its favorability by patients and partners. The reasons why tadalafil is a distinct, efficacious, and favorable treatment in the clinical armamentarium for the treatment of ED will be clear at the conclusion of this review.
Erectile dysfunction
ED is the inability to achieve and maintain an erection sufficient to permit satisfactory sexual intercourse (NIH 1993). Affecting 150 million men worldwide, ED is growing rapidly, and its prevalence is expected to double to greater than 300 million men worldwide over the next 20 years (McKinlay 2000). According to data extrapolated from the Massachusetts Male Aging Study, the incidence of ED is increasing at an astronomical rate, with an expected incidence of over 600,000 new cases per year in white men aged 40 to 69 alone (Johannes et al 2000). Despite its growing incidence, which is partly a result of the sexual awakening stimulated by the new pharmacologic therapies, ED remains underdiagnosed, with millions of men worldwide never coming to medical attention because of the sensitivity of the issue.
The pathophysiology of ED has a variety of etiologies including psychological, hormonal, neurogenic, vasculogenic, drug-induced, or cavernosal impairment from any of the aforementioned factors (Lue 2000). The etiologies are frequently categorized into psychogenic, organic (hormonal, neurogenic, vasculogenic, drug-induced, and cavernosal impairment), or the most common type of ED which is a mixed psychogenic and organic type (Lue 2000).
The pharmacologic evolution that has occurred over the past several decades has directed the current understanding of the pathophysiology of the disease process. Until the 1980s, the treatment options were limited to psychosexual therapy and placement of penile implants, and through that time it was believed that ED was primarily due to psychogenic causes. With the introduction of prostaglandin intracavernosal injections in the late 1980s, the vasculogenic etiologies of ED became better understood, and the connection between late onset hypogonadism and ED led to research elucidating the hormonal etiologies of the condition (Kaminetsky 2008). Finally, the new oral treatment with PDE5 inhibitors has led to the current understanding of the pathophysiology of ED as well as the physiology of erection.
The physiology of erection and the role of phosphodiesterase type 5
A penile erection is a transformation of erectile tissue and vasculature from a state of minimally-perfused flaccidity into an engorged state. It is mediated by a multifaceted succession of neural and vascular components, coupled with hormonal and psychological factors. Through complex neural pathways consisting of somatic sensory afferent (pudendal) and autonomic (cavernous) nerves, as well as supraspinal structures including the medial preoptic area and paraventricular nucleus of the hypothalamus, sexual arousal stimulates the release of neurotransmitters that initiate erection (Carson and Lue 2005).
The primary erectile-mediating neurotransmitter is nitric oxide (NO), a short-lived, gaseous mediator. NO is synthesized in nonadrenergic, noncholinergic fashion by neuronal nitric oxide synthase (nNOS) in the cavernous nerves to initiate erection and by endothelial nitric oxide synthase (eNOS) in the endothelium to maintain erection during sexual stimulation (Hurt et al 2002). NO passively crosses the cell membrane and activates soluble guanylyl cyclase (sGC) upon entering smooth muscle cytoplasm, which in turn increases the production of cyclic guanosine monophosphate (cGMP) by converting it from guanosine triphosphate (GTP) (Lincoln and Cornwell 1991). The increased concentration of cGMP activates protein kinase G, also called cGMP-dependent kinase, which through phosphorylation of ion channels opens potassium channels and inhibits calcium channels. The resultant decrease in cytosolic calcium concentration favors smooth muscle relaxation (Dean and Lue 2005).
The vascular smooth muscle relaxation simultaneously vasodilates arterioles and trabecular smooth muscle sinusoids within erectile tissue to increase penile blood flow. Subsequently, compression of the subtunical venules against the tunica albuginea occludes venous outflow. During the final phase of rigid erection, robust contraction of the ischiocavernous muscles constricts the base of the blood-filled corpora cavernosa, causing the penis to become even harder. Arterial inflow and venous outflow are temporarily occluded during this phase, and the intracavernous pressure can approach several hundred millimeters of mercury (Lue 2000).
Detumescence results from sympathetic discharge with ejaculation, as well as at the molecular level with the cessation of NO release from the endothelium and the breakdown of cGMP and other secondary messengers by various phosphodiesterase (PDE) enzymes (Lue 2000). PDE is an enzyme that 50 years ago was discovered to block the activity of the second messenger cyclic adenosine 3’,5’-monophosphate (cAMP) in animal models (Sutherland and Rall 1958). The PDE superfamily includes 11 families, PDE1 to PDE11, on 21 unique genes (Lin et al 2003). They are distributed throughout various tissues, primarily in vascular, visceral, and pulmonary smooth muscle, and they regulate physiologic functions in multiple organ systems (Carson and Lue 2005). In the penis, PDE acts to breakdown cGMP. Despite the observation that every family of PDE except PDE6 has been found in the corpus cavernosum, PDE5 is the most abundant PDE family in the penis (Carson 2007). By inhibiting the breakdown of cGMP, PDE5 inhibitors create increased bioavailability of cGMP, which both facilitates and potentiates the NO-mediated relaxation of erectile smooth muscle with sexual stimulation.
Caffeine and theophylline were two of the first drugs found to inhibit the PDE enzyme decades ago (Butcher and Sutherland 1962). Over the past 30 years, inhibitors of several families of PDE have been developed to treat a number of diseases. These include the PDE3 inhibitors milrinone and amrinone developed in the 1980s for heart failure, a PDE4 inhibitor cilostazol developed for claudication, and the anti-platelet drug dipyridamole that inhibits PDE8, PDE9, and PDE5 (Carson and Lue 2005). Papaverine was the first PDE inhibitor used in the treatment of ED, and it is still used in practice today. Administered as an intracorporal injection, papaverine is a non-selective inhibitor of PDE3 and its isoforms (Carson 2007). Originally investigated as a treatment for angina pectoris, the first oral PDE5 inhibitor, sildenafil, was fortuitously discovered to produce erections in study participants. It was later released in 1998 as the first oral treatment for ED, and this was followed in 2003 by the release of two more PDE5 inhibitors, vardenafil and tadalafil.
History of the development of tadalafil
The Bothell, Washington-based pharmacologic research company ICOS Corporation was started in 1990, and it began the initial cardiovascular testing of a PDE5 inhibitor called IC351 in 1993. Meanwhile, sildenafil citrate (sildenafil) was discovered to cause improved erectile function as a side effect in a trial testing its efficacy for the treatment of angina pectoris in 1994. IC351 was patented that year, and phase I clinical trials began in 1995. Two years later, phase II clinical trials began on patients with ED.
The same year that the FDA approved sildenafil as the first PDE5 inhibitor for the treatment of ED, Eli Lilly and Company joined the ICOS Corporation to form Lilly ICOS LLC in 1998 to expand the marketing venture of the newest PDE5 inhibitor. Tadalafil was officially born in the year 2000 when a new drug application was put forth for IC351 with the generic name tadalafil and trade name Cialis.
In May 2002, the first reports regarding the efficacy and duration of action of tadalafil for ED were presented at the 97th Annual Meeting of the American Urological Association (AUA) in Orlando, Florida, USA. Brock and colleagues presented their initial data supporting the efficacy and safety of tadalafil (Brock et al 2002b), and Porst and colleagues presented that tadalafil is efficacious for up to 36 hours (Porst et al 2002). Brock’s data were published later that year in a landmark integrative analysis of five randomized controlled trials of tadalafil that ultimately led to the approval of the drug (Brock et al 2002a). Tadalafil was approved for use in Europe in late 2002, and on November 21, 2003, tadalafil was approved by the FDA for use in the United States.
Tadalafil pharmacology
The three available PDE5 inhibitors share a similar mechanism of action, but they have structural, pharmacologic, and clinical differences. The molecular structure of tadalafil is available in Figure 1 . Tadalafil’s molecular structure is different than the similar structures of sildenafil and vardenafil. All three have a heterocyclic nitrogen-containing doublering system, with a central ring that is analogous to that of cGMP and allows for competitive binding of the drug with PDE5 at the catalytic site (Francis et al 2001). Tadalafil is different in that it is a β-carboline-type PDE5 inhibitor with a piperazinedione ring formed from a modification of the hydantoin ring of sildenafil (Francis et al 2001).
Phosphodiesterase selectivity
Tadalafil is at least 9000 times more selective for PDE5 than most of the other families of PDEs, with the exception of PDE11 (Briganti et al 2005). PDE11 is found in the testes and prostate; however, despite partial inhibition of PDE11 by tadalafil at therapeutic doses, clinical significance of this observation has yet to be fully understood (ICOS 2008). A study by Hellstrom and colleagues concluded that there are no harmful effects of tadalafil on spermatogenesis or testicular function (Hellstrom et al 2003).
The PDE enzyme responsible for phototransduction in the retina, PDE6, is inhibited to some degree by sildenafil and vardenafil, but not tadalafil. The inhibition of PDE6 explains the side effect of blue vision experienced by some patients with these two drugs, as PDE6 inhibition in the retina causes impairment of blue-green color discrimination (Carson 2007). Slightly less common with vardenafil, altered vision is a reported side effect for 11% of men taking sildenafil 100 mg (Montorsi et al 1999). Compared with sildenafil and vardenafil, tadalafil is much less inhibitory for PDE6, and it is over 700 times more potent for PDE5 than PDE6 (ICOS 2008). For this reason, tadalafil has less than 0.1% occurrence of vision abnormalities (Brock et al 2002; Carson et al 2004a).
Dosing and absorption
Recommended starting doses of tadalafil are 10 mg for on-demand dosing and 2.5 mg for once-daily dosing, and these doses can then be titrated up or down according to the efficacy and tolerability (ICOS 2008). It is absorbed as a low-solubility and high-permeability, or Class 2, drug within the FDA Biopharmaceuticals Classification System (Gupta et al 2005). With oral ingestion, after first-pass metabolism, tadalafil is approximately 80% bioavailable, compared to 40% and 15% with sildenafil and vardenafil, respectively (Francis and Corbin 2003). Tadalafil has the slowest absorption of the available PDE5 inhibitors with a mean of 2 hours to reach its maximum concentration, compared with about 50 minutes for sildenafil and vardenafil (Briganti et al 2005). The onset of action of tadalafil may occur in as early as 15 minutes of dosing, although successful erections occur in fewer than 40% of men at this time point (Brock et al 2002; Porst et al 2003). It is not advisable to counsel patients that the drug effect may be seen in as early as 15 minutes because it may take up to 2 hours for a response in the majority of men. This may only create performance anxiety or loss of confidence in the treatment, leading to treatment failure (Carson 2007).
Differences in gastrointestinal absorption with fatty meals explain the varying peak plasma concentration (Tmax) among the three PDE5 inhibitors (Corbin and Francis 2002). While sildenafil and vardenafil both have decreased absorption when taken with a fatty meal, which may increase the rate of treatment failure, the absorption of tadalafil is unaffected by fatty meals or alcohol consumption (Francis and Corbin 2003). This unique pharmacokinetic trait for tadalafil is a result of slower absorption and longer half-life, and it can therefore be taken with meals or alcohol without a decrease in efficacy (Carson 2007).
Duration of action
Among the three PDE5 inhibitors, the half-life and thus duration of action is the pharmacologic parameter that is the most strikingly dissimilar. The half-life of tadalafil is 17.5 hours in normal healthy men and 21.6 hours in elderly men, while the half-lives of sildenafil and vardenafil are similar at 4 hours (Francis and Corbin 2003). This longer half-life provides a therapeutic window of 36 hours for tadalafil (Porst et al 2003).
The risks of a longer half-life have yet to be completely elucidated, but there does not appear to be any higher mortality with tadalafil compared to the other PDE5 inhibitors. One difference resulting from the half-life disparity is that emergent treatment with nitrate medications must be deferred for at least 48 hours after ingestion of tadalafil, compared with 24 hours for sildenafil and vardenafil (Kloner et al 2003a).
Pharmacologic treatments for ED prior to tadalafil, including prostaglandin intracavernosal injections or intra-urethral pellets, as well as the other two PDE5 inhibitors, all have a short half-life. For this reason, historically it has been necessary for ED treatments to be dosed immediately prior to attempting intercourse. A drawback to this necessary dosing schedule for short half-life ED treatments is potential performance anxiety. The therapeutic window of 36 hours of tadalafil allows the patient more freedom to choose the timing and setting of the sexual encounter with his partner.
Other benefits of tadalafil’s longer half-life are still being explored. The FDA recently expanded the indications for tadalafil to once-daily dosing for the treatment of ED. The longer half-life provides a steady state of serum drug concentration with low dose, once-daily administration, a benefit not shared with the other two PDE5 inhibitors (McMahon 2004; McMahon 2005; Porst et al 2006; Porst et al 2008; Rajfer et al 2007).
Metabolism and excretion
The metabolism of tadalafil is via the hepatic enzyme cytochrome P450 34A (CYP34A) to a catechol metabolite, which undergoes further metabolism to its primary circulating metabolite, methylcatechol glucuronide, a 10,000-fold less potent molecule than tadalafil (Gupta et al 2005). The CYP34A metabolism pathway of tadalafil was verified with studies using a CYP34A inhibitor ketoconazole and a CYP34A inducer rifampin (Kostis et al 2005). Tadalafil is excreted largely as metabolites, approximately two-thirds into the feces and one-third into the urine (ICOS 2008).
Adverse events
A large study of 2102 men from 11 different multicentered, randomized, double-blind, placebo controlled trials of tadalafil reported the drug is well tolerated overall (Carson et al 2004a). Fifty-one percent of men using tadalafil 20 mg had at least one adverse event (AE), but only 3.2% discontinued treatment. The most common AEs reported with the 20 mg dose were headache (15%), dyspepsia (8%), and back pain (5%) ( Table 1 ) (Carson et al 2004a). Most AEs are a result of vasodilation of vascular beds other than in the penis.
Table 1
Most common tadalafil treatment-emergent adverse events
| Safety variable | Placebo (n = 638) | Tadalafil 10 mg (n = 321) | Tadalafil 20 mg (n = 1143) |
|---|---|---|---|
| Subjects with ≥1 treatment emergent AE | 247 (39%) | 185 (58%) | 577 (51%) |
| Discontinuation for AE | 8 (1.3%) | 5 (1.6%) | 36 (3.2%) |
| Most common treatment emergent AEs | |||
| Headache | 30 (5%) | 38 (12%) | 173 (15%) |
| Dyspepsia | 7 (1%) | 23 (7%) | 90 (8%) |
| Back pain | 15 (2%) | 20 (6%) | 60 (5%) |
| Nasopharyngitis | 24 (4%) | 26 (8%) | 23 (2%) |
| Myalgia | 6 (1%) | 16 (5%) | 33 (3%) |
| Flushing | 8 (1%) | 10 (3%) | 39 (3%) |
| Nasal congestion | 4 (1%) | 11 (3%) | 28 (2%) |
| Pain in limb | 5 (1%) | 10 (3%) | 31 (3%) |
Data from an integrated analysis of 2102 patients in 11 randomized, double-blind, placebo-controlled trials. Adapted with permission from Carson CC, Rajfer J, Eardley I, et al 2004a. The efficacy and safety of tadalafil: an update. BJU Int, 93:1276–81. Copyright © 2004 Wiley-Blackwell.
Abbreviation: AE, adverse events.
Although frequently asked about by patients, priapism is rarely associated with PDE5 inhibitors. Several case reports have found an extraordinarily uncommon link between sildenafil and priapism, and only a single case report exists in the literature implicating tadalafil with priapism (King et al 2005). PDE5 inhibitors including tadalafil may actually have some benefit in preventing recurrent, or stuttering, priapism. A small series of 4 men with stuttering priapism were administered daily PDE5 inhibitors, one case of which used tadalafil, and all four men had decreases in priapism recurrences (Burnett et al 2006).
Safety and drug interactions
As with every PDE5 inhibitor, tadalafil is absolutely contraindicated with nitrate medications for angina pectoris secondary to the potentiated hypotensive effect the drugs can have together. Because of the longer half-life, treatment with nitrate medications must be deferred for at least 48 hours after ingestion of tadalafil, compared with 24 hours for sildenafil and vardenafil (Kloner et al 2003a).
The cardiac effects of PDE5 inhibitors have received considerable attention because the drugs were originally studied as a treatment for angina pectoris secondary to their action of smooth muscle relaxation. All of the PDE5 inhibitor trials have tediously monitored study participants’ vital signs, particularly heart rate and blood pressure, monitored drug effects on cardiac electrophysiology, and tabulated the numbers of cardiovascular adverse events (Carson 2005).
In a review of more than 4000 patients from over 60 studies, data reported by Kloner and co-workers contended that tadalafil does cause small changes in blood pressure secondary to its vasodilatory properties, but that the changes were not clinically meaningful (Kloner et al 2003b). In a separate, more recent study, the same author retrospectively reviewed serious cardiovascular treatment-emergent adverse events reported in 36 tadalafil trials and found that the incidence of these events were comparable among patients taking tadalafil as needed, 3 times per week, once-daily, and placebo (Kloner et al 2006).
The Second Princeton Consensus Conference, an expert conference on sexual dysfunction and cardiac risk, convened in 2004 to develop practice guidelines for the management of ED in patients with significant cardiac risk. After reviewing all available literature, the Second Princeton Consensus Conference not only determined that patients with significant cardiac risk factors did not exhibit worsening ischemia or unstable hemodynamics while taking PDE5 inhibitors, but also that cardiovascular function was actually improved by PDE5 inhibitors in some studies (Kostis et al 2005). Although reported with vardenafil, prolongation of the QTc interval is not observed with tadalafil (Kloner 2004).
Tadalafil does not cause increased hypotension or orthostatic hypotension in men that are also taking multiple antihypertensive medications, and it is safe and well tolerated in this population (Kloner et al 2003c). The primary exception is with α-blockers. Frequently prescribed for men with benign prostatic hyperplasia (BPH) as well as for men with hypertension, α-blockers share a similar mechanism of action with PDE5 inhibitors through peripheral vasodilation and may have more of an additive effect than other antihypertensives. Recently, the FDA changed its previous recommendation from contraindicated co-administration to precautionary co-administration (Kostis et al 2005). In a study of the blood pressure effects of tadalafil coupled with either of two α-blockers, doxazosin (Cardura ® ; Pfizer, New York, NY, USA) and tamsulosin (Flomax ® ; Boehringer Ingelheim, Ridgefield, CT, USA), the hypotensive effect of doxazosin was exaggerated by almost 10 mmHg with tadalafil while there was no change in blood pressure with tamsulosin and tadalafil (Kloner et al 2004). The authors concluded tamsulosin to be a safe α-blocker to administer concurrently with tadalafil (Kloner et al 2004). Until more studies are available, precaution is recommended with the co-administration of tadalafil with any α-blocker, including mixed α-blockers such as labetalol and carvedilol (Kostis et al 2005; ICOS 2008).
Because of its metabolism through the cytochrome P450 pathway, tadalafil is susceptible to changes in serum concentration by other drugs that inhibit or promote its metabolism. Inhibitors of the CYP34A enzyme include ketoconazole, erythromycin, grapefruit juice, and protease inhibitors, and co-administration of these drugs with tadalafil may increase serum concentrations of tadalafil. On the contrary, co-administration with inducers of CYP34A including rifampin, carbamazepine, phenytoin, and phenobarbital, would require a larger dose of tadalafil for a similar clinical effect (Kostis et al 2005).
Efficacy
Tadalafil is an efficacious treatment for ED of all severities and etiologies. The largest data series exist for the general ED population with mild to moderate ED (Brock et al 2002a; Carson et al 2004a), but tadalafil has also been shown to be an effective treatment for difficult-to-treat ED such as more severe, organic ED evaluated in tertiary care centers (Carson et al 2005a), ED secondary to diabetes mellitus (Saenz de Tejada et al 2002; Fonseca et al 2004), and ED resulting from prostate cancer treatments such as prostatectomy (Montorsi et al 2004; Carson et al 2005b) and radiotherapy (Incrocci et al 2007).
Several recent studies have shown a significant treatment benefit from once-daily, low-dose tadalafil (McMahon 2004; McMahon 2005; Porst et al 2006; Rajfer et al 2007; Hatzichristou et al 2008; Porst et al 2008). These studies have lead to the most recent development in the treatment of ED, once-daily tadalafil for ED, which was FDA-approved in 2008.
Background on outcome measures
Although the effectiveness of PDE5 inhibitors on such intermediate objective outcomes as penile rigidity through penile plethysmography with the RigiScan device (Dacomed Corporation, Minneapolis, MN, USA) has been studied, measuring the therapeutic effectiveness of tadalafil is more accurately defined through an integration of the patient’s reported treatment response and tolerability with the reported satisfaction of both the patient and his partner (Carson et al 2004b). Despite their intrinsically subjective nature, validated questionnaires are the preferred major outcome measure of treatment effectiveness of tadalafil and other PDE5 inhibitors.
The International Index of Erectile Function (IIEF) was developed by Rosen and colleagues in 1997 as a multidimensional, 15-item, self-administered questionnaire with the goal of assessing five domains of male sexual function including erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction (Rosen et al 1997). The erectile function domain of the IIEF (IIEF-EF) contains 6 questions which the patient answers on a scale from 1 (never or almost never) to 5 (almost always or always), providing a total score of 6 to 30 points. The questions concern erectile frequency, firmness, penetration ability, maintenance frequency, maintenance ability, and erection confidence (Rosen et al 1997). Based on a controlled study of 1151 men taking sildenafil in order to establish cutoff scores for the IIEF-EF, a score of 26 or greater is defined as normal function, mild ED is a score from 22 to 25, mild to moderate ED 17 to 21, moderate ED 11 to 16, and severe ED 6 to 10 (Cappelleri et al 1999).
The Sexual Encounter Profile (SEP) is a patient diary that has a series of questions that the patient answers after each sexual encounter. The second question in the SEP diary (SEP-Q2) is a measure of penetration, “Were you able to insert your penis into your partner’s vagina?” The third question of the SEP (SEP-Q3) is a true measure of successful intercourse, “Did your erection last long enough for you to have successful intercourse?” In tadalafil trials including data analyses of the SEP, the SEP-Q2 and SEP-Q3 are most often reported.
Less often but still occasionally reported in the literature is the Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS) score, which more reliably assesses the patient and his partner’s satisfaction with ED treatments and explores the impact of patient and partner satisfaction on treatment continuation (Althof et al 1999). Graded on a scale of 1 to 100, a score of 50 or higher indicates treatment satisfaction (Carson and Lue 2005).
In addition to the IIEF and the SEP, many tadalafil trials report a global overall assessment as provided by the patient, known as a Global Assessment Question (GAQ). It is a summary question to assess global improvement and to provide an indirect estimate of patient satisfaction. A frequently reported GAQ in tadalafil trials is, “Did the treatment improve your erections?”
The general ED population
The most frequently referenced study supporting the efficacy of tadalafil is an integrated analysis of 5 randomized, double-blind, placebo controlled, multicentered phase III trials from 1112 men at 74 centers worldwide (Brock et al 2002a). The average age was 59, and the etiology of ED was 61% organic, 9% psychogenic, and 31% mixed. The ED severity at baseline was mild in 41%, moderate in 23%, and severe in 36%. Subjects were randomized to placebo or tadalafil at doses from 2.5 mg to 20 mg and instructed to self-administer a dose before initiating intercourse up to once daily. IIEF-EF, SEP, and GAQ scores were assessed at baseline and at 12 weeks (Brock et al 2002a).
In an update to Brock and colleagues’ study, Carson and associates performed another integrated analysis on all available tadalafil trials, including the original 1112 men in the 5 trials from Brock and colleagues’ original study, plus an additional 1215 men from 6 more recently completed trials (Carson et al 2004a). The same study design, safety measures, and statistical analysis were performed, except patients who received 2.5 mg and 5 mg doses in two of the earlier studies that were included in the analysis by Brock and colleagues were not included by Carson and associates. Only doses of 10 mg and 20 mg of tadalafil were compared with placebo. The average age was 56, and the etiology of ED was 58% organic, 12% psychogenic, and 31% mixed. The ED severity at baseline was mild in 36%, moderate in 27%, and severe in 33%. Comorbidities included 29% of men with hypertension, 16% with hyperlipidemia, 20% with diabetes mellitus, and 5% with coronary artery disease (Carson et al 2004a).
IIEF-EF, SEP-Q2, and SEP-Q3 improvements from baseline to 12 weeks with on-demand tadalafil.
a Erectile function domain score of the International Index of Erectile Function.
b Question 2 from the Sexual Encounter Profile diary, “Were you able to insert your penis into your partner’s vagina?”
c Question 3 from the Sexual Encounter Profile diary, “Did your erection last long enough for you to have successful intercourse?”
Data from an integrated analysis of 2102 patients in 11 randomized, double-blind, placebo-controlled trials. Adapted with permission from Carson CC, Rajfer J, Eardley I, et al 2004a. The efficacy and safety of tadalafil: an update. BJU Int, 93:1276–81. Copyright © 2004 Wiley-Blackwell.
Difficult-to-treat ED
In the two trials mentioned in the previous section, the patient population was largely a primary care population of relatively healthy men with ED of varying severities and etiologies. To the contrary, a multicentered, randomized, double-blind, placebo controlled trial evaluated how tadalafil 20 mg improved ED in men presenting to tertiary care centers with more severe, organic ED and with more comorbid medical conditions than previous studies (Carson et al 2005a).
One hundred and ninety-five men were allocated to receive tadalafil 20 mg or placebo up to once daily for 12 weeks with simplified dosing instructions (Carson et al 2005a). Efficacy was assessed using the IIEF-EF, the SEP, and a GAQ, and the EDITS was provided at the end of the trial to assess satisfaction of the patient and his partner. The mean baseline IIEF-EF was 13, with 33% of participants reporting mild ED, 23% with moderate ED, and 51% with severe ED at baseline. Organic ED predominated with 81%, followed by 3% of men with psychogenic ED, and 16% with mixed type. Comorbidities included 41% with hypertension, 36% with hyperlipidemia, 21% with diabetes mellitus, and 8% with coronary artery disease (Carson et al 2005a).
ED secondary to diabetes mellitus
ED is a common problem afflicting over a third of all men with diabetes mellitus type 2, and diabetes is independently responsible for a 3- to 4-fold increase in the risk of ED according to a survey of 1460 diabetic men (De Barardis et al 2003). ED is strongly related to the severity of diabetes, with a higher incidence in patients with a long history of diabetes, patients using insulin, and patients with microvascular complications of diabetes (De Berardis et al 2003). Diabetic men with ED also have significantly worse disease-specific health-related quality of life (Penson et al 2003).
In the largest original, prospective trial of tadalafil in diabetic men, Sáenz de Tejada and co-workers reported that tadalafil significantly improves ED and is well tolerated in this population (Sáenz de Tejada et al 2002). A total of 216 men with type 1 or type 2 diabetes mellitus were randomized to placebo, tadalafil 10 mg, or tadalafil 20 mg to be taken on-demand up to once daily for 12 weeks. The IIEF-EF, the SEP, and a GAQ were used to evaluate efficacy. The average age was 56, and the duration of diabetes was 12 years on average. Ninety-one percent of men had type 2 diabetes while 9% had type 1 diabetes, 22% had microvascular complications, and over 81% of men had poorly controlled diabetes with a hemoglobin A1c of greater than 7.0%. The baseline IIEF-EF was 12, corresponding to moderate severity ED (Sáenz de Tejada et al 2002).
Results from a large, retrospective analysis of 12 randomized controlled trials of tadalafil including over 600 diabetic men parallels the data reported by Sáenz de Tejada (Fonseca et al 2004). The trials included in this retrospective analysis were many of the same trials analyzed in other integrated analyses reported here in this review. The average age was 57, and diabetic men had more severe ED than patients without diabetes at baseline with a mean IIEF-EF of 12.6 compared to 15 in patients without diabetes (p < 0.001). Comorbid conditions more common in the patients with diabetes included hypertension, hyperlipidemia, and coronary artery disease (Fonseca et al 2004).
ED after prostate cancer treatment
All of the available prostate cancer treatments, including radical prostatectomy, external beam radiation therapy, brachytherapy, cryotherapy, androgen-deprivation therapy, and even active surveillance alone, can result in ED. ED after prostate cancer treatment includes both organic and psychogenic causes, as significant anxiety and depression may result from a diagnosis of prostate cancer, leading to psychogenic ED (Carson et al 2005b).
The most important risk for organic ED after prostate cancer treatment of any type is damage to the cavernosal nerves. Walsh first described the technique for sparing the bilateral neurovascular bundles to better preserve erectile function (Walsh et al 1983), and a bilateral nerve sparing radical prostatectomy (BNSRP) is the surgical standard for prostate cancer today. Approximately 155,000 prostatectomies were performed in the United States in 2005 according to hospital discharge data (DeFrances 2007). Potency in men after open BNSRP has a wide reported range of 10% to 97% in the literature (Talcott et al 1997; Stanford et al 2000; Walsh et al 2000; Menon et al 2005; Penson et al 2008). Of 1288 men who underwent radical prostatectomy as part of the Prostate Cancer Outcomes Study, only 28% had erections sufficient for intercourse at 5 years (Penson et al 2008).
Cavernosal nerve injury during prostate cancer treatment is a neuropraxia resulting in atrophy of the cavernosal smooth muscle and abnormal deposition of collagen into the corpora cavernosa. Cavernosal hypoxia is another contributing factor in the development of fibrosis post-prostatectomy (Raina et al 2008). The smooth muscle atrophy and fibrosis leads to corporal veno-occlusive dysfunction (CVOD), which is recognized as the primary cause of organic ED after prostate cancer treatment (Rambhatla et al 2008).
There are increasing experimental data that increased concentrations of NO and cGMP have an antifibrotic effect on tissues including the tunica albuginea and corporal tissue, supporting a role for tadalafil to halt the pathophysiologic role of CVOD in post-prostate cancer treatment ED. Kovanecz and colleagues have recently studied the effect of once-daily tadalafil on the prevention of fibrosis and CVOD after cavernosal nerve injury in rats (Kovanecz et al 2007). Male rats had either a bilateral cavernosal nerve resection, unilateral cavernosal nerve resection, or a sham operation, and they were then either untreated or given once-daily tadalafil. CVOD was assessed at 45 days with cavernosometry and histopathology. The authors found that tadalafil normalized the increase in penile shaft collagen content, normalized the reduction in corporeal smooth muscle content, and improved the lower collagen type III:I ratio, effectively preventing CVOD and underlying corporal fibrosis after cavernosal nerve damage (Kovanecz et al 2008). Similarly, Vignozzi et al also found that once-daily tadalafil given to rats that have undergone bilateral cavernosal nerve resection reversed the decline in cavernosal smooth muscle to collagen ratio (Vignozzi et al 2006).
In the clinical arena, on-demand dosing of tadalafil has been studied for ED secondary to BNSRP and external-beam radiotherapy (Incrocci et al 2006; Montorsi et al 2004). Mon-torsi and associates evaluated on-demand dosing of tadalafil 20 mg in men with ED following BNSRP in a randomized, double-blind, placebo controlled, multicentered study of 303 men over 12 weeks (Montorsi et al 2004). All men had normal erectile function preoperatively and had undergone a BNSRP 12 to 48 months prior to the study. Primary endpoints were IIEF-EF, SEP-Q2, and SEP-Q3, and secondary endpoints were the GAQ and the EDITS questionnaire. Average age was 60 years, and 93% were white men. Almost two-thirds of the participants had some degree of postoperative tumescence at baseline as defined by reporting the ability “to achieve at least some erection” in over half of their sexual encounters on the SEP-Q1 (Montorsi et al 2004). Among all patients, 62% responded to tadalafil on the GAQ compared with 23% placebo (p < 0.001). Of those with some postoperative potency, 71% responded to tadalafil compared with 24% placebo (p < 0.001). The SEP-Q3 revealed 41% of the total group had successful intercourse compared with 19% with placebo (p < 0.001). Over 50% of men with residual function had successful intercourse compared with 26% placebo (p < 0.001) (Montorsi et al 2004). In this trial, tadalafil was successful in producing erections sufficient for intercourse in men with ED after BNSRP, but it was more efficacious in men with some residual potency after surgery prior to treatment.
Sixty patients with ED after external-beam radiotherapy for prostate cancer were randomized to on-demand tadalafil 20 mg or placebo in a double-blind, placebo controlled, crossover study lasting 12 weeks (Incrocci et al 2006). Efficacy was assessed with the IIEF-EF, SEP, and a GAQ. Average age was higher than most other tadalafil studies at 69 years, the participants’ prostate cancer tumor stages were 37% T1c, 37% T2, and 26% T3, and the baseline mean IIEF-EF score among all men revealed severe ED at 8.4. The IIEF-EF score improved to 17.7 with tadalafil and to 9.5 with placebo (p < 0.0001). The SEP-Q3 data demonstrated 46% of participants successfully had intercourse with tadalafil 20 mg compared with 12% with placebo (p < 0.0001), and 67% of patients taking tadalafil 20 mg responded positively with the GAQ compared with 20% with placebo (p < 0.001) (Incrocci et al 2006). This study was continued as an open-label extension over 6 weeks. Fifty-one of 60 patients (85%) enrolled, who had a higher IIEF-EF score than those who did not enroll (p < 0.05). Tadalafil was equally effective in the double-blind phase as in the open-label phase of the study (Incrocci et al 2007).
Tadalafil and other PDE5 inhibitors dosed both in continuous fashion and on-demand have been proposed for erectile rehabilitation and ED prophylaxis after BNSRP. In a small, prospective, observational study of 27 patients who underwent BNSRP, tadalafil 20 mg was dosed every 3 days beginning on the first postoperative day (Carson et al 2005b). At 6 weeks, 89% of men reported erections, and 50% had successful intercourse. One-hundred percent had erections at 6 months, with 78% reporting successful intercourse (Carson et al 2005b). Padma-Nathan and colleagues reported in a multicentered, placebo-controlled, prospective study of prophylactic sildenafil dosed nightly beginning 4 weeks after BNSRP and continuing for 36 weeks that patients taking prophylactic sildenafil had a 27% return of erectile function compared with only 4% in the placebo group (p <0.05) (Padma-Nathan et al 2008). A randomized, double-blind, multicentered study of early postoperative dosing with vardenafil, dosed either once-daily or on-demand and compared with placebo, in 628 men after BNSRP found that on-demand vardenafil treatment resulted in greater IIEF-EF scores and higher SEP-Q3 response rates than once-daily dosing or placebo (Montorsi et al 2008). Although the efficacy of postoperative treatment with PDE5 inhibitors to prevent CVOD and corporal fibrosis and reduce ED after BNSRP seems clear, it remains uncertain whether a continuous or an on-demand dosing strategy is superior in this setting.
Once-daily dosing
The latest innovation in the treatment of ED has arrived in the form of a simpler, once-daily dosing schedule for tadalafil, unlinking the temporal association of the medication and the sexual encounter. The unnatural process of taking a medication just prior to sex is a negative aspect of ED treatment for many patients (Hanson-Divers et al 1998). The 17.5 hour half-life of tadalafil lends itself to daily dosing because steady-state plasma concentrations are attained within five days of initiating daily dosing (Forgue et al 2006). Additionally, at steady-state concentration, the daily exposure is 1.6-fold greater than the same dose taken intermittently (Forgue et al 2006). Therefore, after 5 days of once-daily dosing, the plasma concentration of tadalafil achieved with a 2.5 mg and 5 mg dose is 4 mg and 8 mg, respectively. The FDA announced approval for once-daily dosing of tadalafil in January 2008 after a thorough review of the studies outlined below.
McMahon has compared once-daily with on-demand dosing of tadalafil in two independent, open-label studies in Australian men with ED (McMahon 2004; McMahon 2005). In 112 men with ED who were previously unresponsive to on-demand tadalafil, once-daily tadalafil at flexible doses of 10 mg and 20 mg for 12 weeks was provided (McMahon 2004). Baseline IIEF-EF, SEP, and GAQ data were obtained from the cohort prior to any treatment with tadalafil and after the trial of on-demand tadalafil. Changes in the scores from both baselines were assessed after 12 weeks of daily dosing. Patients taking tadalafil 10 mg once-daily improved the IIEF-EF score 12.8 from the no-treatment baseline and 8.2 from the on-demand treatment baseline (p < 0.001). Compared with 42% of men with on-demand tadalafil, 69% of men with once-daily tadalafil reported improved erections at the endpoint (McMahon 2004). McMahon then compared once-daily tadalafil 10 mg with on-demand tadalafil 20 mg in an open-label, parallel-arm, crossover study of 145 Australian men with ED (McMahon 2005). While both dosing strategies were efficacious, the once-daily dosing of tadalafil improved the IIEF-EF score by 11.9, compared to an improvement of 8.3 with the on-demand dosing (p < 0.05). Additionally, compared with 30% at baseline, successful intercourse was reported in the SEP-Q3 in 69% and 84% of patients taking on-demand and once-daily tadalafil, respectively (p < 0.05). Overall, once-daily dosing was preferred by 72% of the patients (McMahon 2005).
The first multicentered, randomized, double-blind, placebo controlled study of once-daily tadalafil enrolled 268 men over 12 weeks and compared tadalafil 5 mg and 10 mg taken once-daily with placebo (Porst et al 2006). The study took place in 20 centers across Europe and South America in men with ED of all severities and etiologies. IIEF-EF, SEP, and GAQ were assessed at baseline and at 12 weeks. The mean IIEF-EF change from baseline at the endpoint was 9.7 and 9.4 for tadalafil 5 mg and tadalafil 10 mg, respectively, compared with 0.9 for placebo (p < 0.001). Successful intercourse was achieved in 67.2% and 72.8%, compared with 36.7% with placebo (p < 0.001). The authors concluded once-daily tadalafil 5 mg or 10 mg significantly improved erectile function in men with ED (Porst et al 2006).
Rajfer and associates performed a similar study of once-daily tadalafil in American men with lower doses over a longer study duration (Rajfer et al 2007). Once-daily tadalafil 2.5 mg, 5 mg, or placebo was dosed over a 24 week period in a randomized, double-blind, placebo controlled, parallel-design study in 287 men evaluated in 15 US centers. Primary endpoints included the change from baseline to 24 weeks in mean IIEF-EF score and the percentage of “yes” responses in SEP-Q2 and SEP-Q3. The IIEF-EF and SEP data are summarized in Figure 3 . The IIEF-EF score had a mean improvement over 24 weeks of 6.0 and 7.0 in the tadalafil 2.5 mg and 5 mg groups, respectively, compared with 1.2 in the placebo group (p < 0.001). Successful intercourse was improved over the 24-week period in 31.2% and 35.1% in the tadalafil groups compared with 9.5% with placebo (p <0.001) (Rajfer et al 2007).
IIEF-EF, SEP-Q2, and SEP-Q3 improvements from baseline to 24 weeks with once-daily tadalafil.
a Erectile function domain score of the International Index of Erectile Function.
b Question 2 from the Sexual Encounter Profile diary, “Were you able to insert your penis into your partner’s vagina?”
c Question 3 from the Sexual Encounter Profile diary, “Did your erection last long enough for you to have successful intercourse?”
Data from a multicenter, randomized, double-blind, placebo-controlled study of 268 men to study once-daily tadalafil over 24 weeks. Adapted with permission from Rajfer J, Aliotta PJ, Steidle CP, et al 2007. Tadalafil dosed once a day in men with erectile dysfunction: a randomized, double-blind, placebo-controlled study in the US. Int J Impot Res, 19:95–103. Copyright © 2007 Macmillan Publishers Ltd.
In open-label extensions of the two randomized, placebo controlled trials of once-daily tadalafil described above, Porst and colleagues have evaluated the long-term safety and efficacy of once-daily tadalafil 5 mg over 1 and 2 years (Porst et al 2008). There were 208 of 234 possible patients (88.9%) who completed the 1-year extension and 139 of 238 possible patients (58.4%) who completed the 2-year extension. Efficacy was measured by changes in the IIEF-EF score, SEP, and the GAQ. The IIEF-EF score improved from baseline by 10.4 and 10.8 in the 1- and 2-year extensions, respectively. After 2 years, 95.7% of patients reported improved erections (Porst et al 2008). Although an impressively positive response, the 2-year GAQ data had a significant dropout rate and likely contained selection bias toward patients who had a positive response.
Once-daily tadalafil has recently been studied in a population of men with ED secondary to diabetes mellitus (Hatzichristou et al 2008). A randomized, double-blind, placebo controlled, multicentered study in North America, Europe, and Australia enrolled 298 diabetic men with ED and randomized them to tadalafil 2.5 mg, 5 mg, or placebo for 12 weeks. IIEF-EF, SEP, and GAQ were measured as primary outcomes. The mean age was 57, 42.6% of patients had severe ED, and comorbid conditions were prevalent and included 54% with hypertension and 43% with dyslipidemia. Eighty-nine percent of patients had diabetes mellitus type 2, average hemoglobin A1c was 7.7%, and glycemic control was good in 39%, fair in 48%, and poor in 13%. Mean changes in IIEF-EF scores were modest, including 4.8 and 4.5 for the tadalafil 2.5 mg and 5 mg groups, respectively, compared with 1.3 for placebo (p < 0.005). Successful intercourse was improved by 25.9% and 25% in the tadalafil groups, versus 8.2% placebo (p < 0.005). In this first study of once-daily tadalafil in diabetic men with ED, 2.5 mg and 5 mg once-daily both produced modest improvements in erectile function in this population (Hatzichristou et al 2008).
New applications
Recent data have indicated a potential association between epidemiological, physiologic, pathophysiologic, and treatment aspects of ED and lower urinary tract symptoms (LUTS) secondary to BPH (McVary and McKenna 2004). The 17.5 hour half-life of tadalafil makes it the most suitable PDE5 inhibitor for once-daily dosing in a trial of LUTS secondary to BPH. McVary and associates reported a multicentered, randomized, double-blind, placebo controlled study of 281 men with LUTS secondary to BPH who were randomly assigned to once-daily tadalafil 5 mg for 6 weeks, followed by dose escalation to 20 mg for 6 weeks, or 12 weeks of placebo. They reported modest decreases in International Prostate Symptom Score (IPSS), with a mean change from baseline to 6 weeks of −2.8 with tadalafil 5 mg versus −1.2 with placebo (p < 0.003), and to 12 weeks of −3.8 with tadalafil 5/20 mg versus −1.7 with placebo (p < 0.001) (McVary et al 2007).
To further examine the efficacy and dose response of tadalafil in men with LUTS secondary to BPH, Roehrborn and colleagues performed a similar multicentered, randomized, double-blind, placebo controlled study with a larger sample size and different tadalafil doses (Roehrborn et al 2008). The 1058 men with LUTS secondary to BPH in the study received once-daily placebo or tadalafil (2.5, 5, 10 or 20 mg) for 12 weeks. The IPSS mean change from baseline to endpoint after 12 weeks was significantly improved, –3.9 for tadalafil 2.5 mg (p <0.015), −4.9 for tadalafil 5 mg (p < 0.001), −5.2 for tadalafil 10 mg (p < 0.001), and −5.2 for tadalafil 20 mg (p < 0.001), compared with −2.3 for placebo. The optimal risk-benefit profile for men with LUTS secondary to BPH was achieved with the tadalafil 5 mg dose (Roehrborn et al 2008).
These observations may be a result of increased cGMP causing a decrease in prostatic muscle tension (Uckert et al 2001), NO effect on the smooth muscle of the bladder, PDE inhibition in the prostate and prostatic urethra, or some other process not yet defined. Further basic science and clinical research is necessary to define the role of tadalafil’s effect on the bladder, the prostate, and LUTS.
Patient preference
There have been several crossover trials that have attempted to compare patient and partner preference between sildenafil and tadalafil. Comparing the PDE5 inhibitors is intrinsically difficult because of the differences in dosing, absorption, duration of action, and other pharmacologic parameters (Carson and Lue 2005). Regardless, consensus results across multiple studies declare tadalafil as preferred over sildenafil by patients and their partners for the treatment of ED.
The Partner’s Preference Study was a randomized, crossover study from a single center comparing tadalafil and sildenafil in 100 couples with the male partner having ED (Conaglen and Conaglen 2008). The couples were randomly assigned to tadalafil or sildenafil for 12 weeks followed by a second phase with 12 weeks of the alternate drug. Men and their partners completed SEP diaries, and the primary outcome data were the female partners’ final interviews during which they provided their preference between the two drugs. Tadalafil was preferred by the female partner in 79.2%, while 15.6% preferred sildenafil. The number of events recorded, timing of the events, and the number of doses were not significantly different between the groups. The female partners reported more relaxed, more satisfying, and longer-lasting sexual experiences with tadalafil as compared with sildenafil (Conaglen and Conaglen 2008).
Conclusion
Tadalafil is a safe, well tolerated, and efficacious treatment for all severities and etiologies of ED. Its efficacy has been proven in numerous clinical trials, and it is effective not only in the general ED population, but also the difficult-to-treat ED population with more severe ED and a greater number of comorbidities. Its most unique characteristic is its long half-life of 17.5 hours, which lends itself to a longer therapeutic window with on-demand dosing and effective steady-state plasma concentrations with once-daily dosing. Taken on-demand or once-daily, tadalafil significantly enhances erectile function. Its newest indication for once-daily dosing disconnects the temporal association of dosing a medication prior to the sexual encounter. These attributes make tadalafil a distinct, efficacious, and favorable treatment in the clinical armamentarium for the treatment of ED.
Acknowledgments
We would like to thank Harriet Ecclesine of Eli Lilly and Company for providing the molecular structure of tadalafil.
The authors have no conflicts of interest to declare.
References
- Althof SE, Corty EW, Levine SB, et al. EDITS: development of questionnaires for evaluating satisfaction with treatments for erectile dysfunction. Urology. 1999; 53 :793–9. [PubMed] [Google Scholar]
- Briganti A, Salonia A, Gallina A, et al. Drug Insight: oral phospho-diesterase type 5 inhibitors for erectile dysfunction. Nat Clin Pract Urol. 2005; 2 :239–47. [PubMed] [Google Scholar]
- Burnett AL, Bivalacqua TJ, Champion HC, et al. Long-term oral phosphodiesterase 5 inhibitor therapy alleviates recurrent priapism. Urology. 2006; 67 :1043–8. [PubMed] [Google Scholar]
- Butcher RW, Sutherland EW. Adenosine 3’,5’-phosphate in biological materials. I. Purification and properties of cyclic 30,50-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3’,5’-phosphate in human urine. J Biol Chem. 1962; 237 :1244–50. [PubMed] [Google Scholar]
- Brock GB, McMahon CG, Chen KK, et al. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: results of integrated analyses. J Urol. 2002a; 168 :1332–6. [PubMed] [Google Scholar]
- Brock G, McMahon C, Point P, et al. Efficacy and safety of tadalafil in men with erectile dysfunction: an integrated analysis of registration trials [abstract] J Urol. 2002b; 167 :178. [Google Scholar]
- Cappelleri JC, Rosen RC, Smith MD, et al. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology. 1999; 54 :346–51. [PubMed] [Google Scholar]
- Carson CC. Cardiac safety in clinical trials of phosphodiesterase 5 inhibitors. Am J Cardiol. 2005; 96 :37M–41M. [PubMed] [Google Scholar]
- Carson CC. Phosphodiesterase type 5 inhibitors: state of the therapeutic class. Urol Clin North Am. 2007; 34 :507–15. [PubMed] [Google Scholar]
- Carson CC, Giuliano F, Goldstein I, et al. The ‘effectiveness’ scale – therapeutic outcome of pharmacologic therapies for ED: an international consensus panel report. Int J Impot Res. 2004b; 16 :207–13. [PubMed] [Google Scholar]
- Carson CC, Hubbard JS, Wallen E. Erectile dysfunction and treatment of carcinoma of the prostate. Curr Urol Rep. 2005b; 6 :461–9. [PubMed] [Google Scholar]
- Carson CC, Lue TF. Phosphodiesterase type 5 inhibitors for erectile dysfunction. BJU Int. 2005; 96 :257–80. [PubMed] [Google Scholar]
- Carson CC, Rajfer J, Eardley I, et al. The efficacy and safety of tadalafil: an update. BJU Int. 2004a; 93 :1276–81. [PubMed] [Google Scholar]
- Carson CC, Shabsigh R, Segal S, et al. Efficacy, safety, and treatment satisfaction of tadalafil versus placebo in patients with erectile dysfunction evaluated at tertiary-care academic centers. Urology. 2005a; 65 :353–9. [PubMed] [Google Scholar]
- Conaglen HM, Conaglen JV. Investigating women’s preference for sildenafil or tadalafil use by their partners with erectile dysfunction: the partners’ preference study. J Sex Med. 2008; 5 :1198–207. [PubMed] [Google Scholar]
- Corbin JD, Francis SH. Pharmacology of phosphodiesterase-5 inhibitors. Int J Clin Pract. 2002; 56 :453–9. [PubMed] [Google Scholar]
- DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat. 2007; 13 :1–209. [PubMed] [Google Scholar]
- De Berardis G, Pellegrini F, Franciosi M, et al. Identifying patients with type 2 diabetes with a higher likelihood of erectile dysfunction: the role of the interaction between clinical and psychological factors. J Urol. 2003; 169 :1422–8. [PubMed] [Google Scholar]
- Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005; 32 :379–95. [PMC free article] [PubMed] [Google Scholar]
- Eardley I, Mirone V, Montorsi F, et al. An open-label, multicentre, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in men naïve to phosphodiesterase 5 inhibitor therapy. BJU Int. 2005; 96 :1323–32. [PubMed] [Google Scholar]
- Francis SH, Corbin JD. Molecular mechanisms and pharmacokinetics of phosphodiesterase-5 antagonists. Curr Urol Rep. 2003; 4 :457–65. [PubMed] [Google Scholar]
- Francis SH, Turko IV, Corbin JD. Cyclic nucleotide phosphodiesterases: relating structure and function. Prog Nucleic Acid Res Mol Biol. 2001; 65 :1–52. [PubMed] [Google Scholar]
- Forgue ST, Patterson BE, Bedding AW, et al. Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2006; 61 :280–8. [PMC free article] [PubMed] [Google Scholar]
- Fonseca V, Seftel A, Denne J, et al. Impact of diabetes mellitus on the severity of erectile dysfunction and response to treatment: analysis of data from tadalafil clinical trials. Diabetologia. 2004; 47 :1914–23. [PubMed] [Google Scholar]
- Govier F, Potempa AJ, Kaufman J, et al. A multicenter, randomized, double-blind, crossover study of patient preference for tadalafil 20 mg or sildenafil citrate 50 mg during initiation of treatment for erectile dysfunction. Clin Ther. 2003; 25 :2709–23. [PubMed] [Google Scholar]
- Gupta M, Kovar A, Meibohm B. The clinical pharmacokinetics of phosphodiesterase-5 inhibitors for erectile dysfunction. J Clin Pharmacol. 2005; 45 :987–1003. [PubMed] [Google Scholar]
- Hanson-Divers C, Jackson SE, Lue TF, et al. Health outcomes variables important to patients in the treatment of erectile dysfunction. J Urol. 1998; 159 :1541–7. [PubMed] [Google Scholar]
- Hatzichristou D, Gambla M, Rubio-Aurioles E, et al. Efficacy of tadalafil once daily in men with diabetes mellitus and erectile dysfunction. Diabet Med. 2008; 25 :138–46. [PubMed] [Google Scholar]
- Hellstrom WJ, Overstreet JW, Yu A, et al. Tadalafil has no detrimental effect on human spermatogenesis or reproductive hormones. J Urol. 2003; 170 :887–91. [PubMed] [Google Scholar]
- Hurt KJ, Musicki B, Palese MA, et al. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci U S A. 2002; 99 :4061–66. [PMC free article] [PubMed] [Google Scholar]
- ICOS 2008 Tadalafil (Cialis) US prescribing information Accessed June 25, 2008. URL: http://www.lilly.com/us/cialis-pi.pdf
- Incrocci L, Slagter C, Slob AK, et al. A randomized, double-blind, placebo-controlled, cross-over study to assess the efficacy of tadalafil (Cialis) in the treatment of erectile dysfunction following three-dimensional conformal external-beam radiotherapy for prostatic carcinoma. Int J Radiat Oncol Biol Phys. 2006; 66 :439–44. [PubMed] [Google Scholar]
- Incrocci L, Slob AK, Hop WC. Tadalafil (Cialis) and erectile dysfunction after radiotherapy for prostate cancer: an open-label extension of a blinded trial. Urology. 2007; 70 :1190–3. [PubMed] [Google Scholar]
- Johannes CB, Araujo AB, Feldman HA, et al. Incidence of erectile dysfunction in men 40–69 years old: longitudinal results from the Massachusetts Male Aging Study. J Urol. 2000; 163 :460–3. [PubMed] [Google Scholar]
- Kaminetsky J. Epidemiology and pathophysiology of male sexual dysfunction. Int J Impot Res. 2008; 20 :S3–10. [PubMed] [Google Scholar]
- King SH, Hallock M, Strote J, et al. Tadalafil-associated priapism. Urology. 2005; 66 :432. [PubMed] [Google Scholar]
- Kloner RA. Cardiovascular effects of the 3 phosphodiesterase-5 inhibitors approved for the treatment of erectile dysfunction. Circulation. 2004; 110 :3149–55. [PubMed] [Google Scholar]
- Kloner RA, Hutter AM, Emmick JT. Time course of the interaction between Tadalafil and Nitrates. J Am Coll Cardiol. 2003a; 42 :1885–60. [PubMed] [Google Scholar]
- Kloner RA, Jackson G, Emmick JT, et al. Interaction between the phosphodiesterase 5 inhibitor, tadalafil and 2 alpha-blockers, doxazosin and tamsulosin in healthy normotensive men. J Urol. 2004; 172 :1935–40. [PubMed] [Google Scholar]
- Kloner RA, Jackson G, Hutter AM, et al. Cardiovascular safety update of Tadalafil: retrospective analysis of data from placebo-controlled and open-label clinical trials of Tadalafil with as needed, three times-per-week or once-a-day dosing. Am J Cardiol. 2006; 97 :1778–84. [PubMed] [Google Scholar]
- Kloner RA, Mitchell M, Emmick JT. Cardiovascular effects of tadalafil. Am J Cardiol. 2003b; 92 :37M–46M. [PubMed] [Google Scholar]
- Kloner RA, Mitchell M, Emmick JT. Cardiovascular effects of tadalafil in patients on common antihypertensive therapies. Am J Cardiol. 2003c; 92 :47M–57M. [PubMed] [Google Scholar]
- Kostis JB, Jackson G, Rosen R, et al. Sexual dysfunction and cardiac risk (the Second Princeton Consensus Conference) Am J Cardiol. 2005; 96 :313–21. [PubMed] [Google Scholar]
- Kovanecz I, Rambhatla A, Ferrini MG, et al. Chronic daily tadalafil prevents the corporal fibrosis and veno-occlusive dysfunction that occurs after cavernosal nerve resection. BJU Int. 2008; 101 :203–10. [PubMed] [Google Scholar]
- Krane RJ. Sexual Function and Dysfunction. In: Walsh Gittes, Perlmutter Stamey., editors. Campbell’s Urology. 5th ed. Vol. 1. Philadelphia: W.B. Saunders Company; 1986. p. 731. [Google Scholar]
- Lin CS, Xin ZC, Lin G, et al. Phosphodiesterases as therapeutic targets. Urology. 2003; 61 :685–91. [PubMed] [Google Scholar]
- Lincoln TM, Cornwell TL. Towards an understanding of the mechanism of action of cyclic AMP and cyclic GMP in smooth muscle relaxation. Blood Vessels. 1991; 28 :129–37. [PubMed] [Google Scholar]
- Lue TF. Erectile dysfunction. N Engl J Med. 2000; 342 :1802–13. [PubMed] [Google Scholar]
- McKinlay JB. The worldwide prevalence and epidemiology of erectile dysfunction. Int J Impot Res. 2000; 12 :S6–S11. [PubMed] [Google Scholar]
- McMahon C. Efficacy and safety of daily tadalafil in men with erectile dysfunction previously unresponsive to on-demand tadalafil. J Sex Med. 2004; 1 :292–300. [PubMed] [Google Scholar]
- McMahon C. Comparison of efficacy, safety, and tolerability of on-demand tadalafil and daily dosed tadalafil for the treatment of erectile dysfunction. J Sex Med. 2005; 2 :415–25. [PubMed] [Google Scholar]
- McVary KT, McKenna KE. The relationship between erectile dysfunction and lower urinary tract symptoms: epidemiological, clinical, and basic science evidence. Curr Urol Rep. 2004; 5 :251–7. [PubMed] [Google Scholar]
- McVary KT, Roehrborn CG, Kaminetsky JC, et al. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2007; 177 :1401–7. [PubMed] [Google Scholar]
- Menon M, Kaul S, Bhandari A, et al. Potency following robotic radical prostatectomy: a questionnaire based analysis of outcomes after conventional nerve sparing and prostatic fascia sparing techniques. J Urol. 2005; 174 :2291–6. [PubMed] [Google Scholar]
- Montorsi F, Brock G, Lee J et al. Effect of Nightly versus On-Demand Vardenafil on Recovery of Erectile Function in Men Following Bilateral Nerve-Sparing Radical Prostatectomy. Eur Urol. 2008 Jul 9; [Epub ahead of print] [PubMed] [Google Scholar]
- Montorsi F, McDermott TE, Morgan R, et al. Efficacy and safety of fixed-dose oral sildenafil in treatment of erectile dysfunction of various etiologies. Urology. 1999; 55 :1011–8. [PubMed] [Google Scholar]
- Montorsi F, Nathan HP, McCullough A, et al. Tadalafil in the treatment of erectile dysfunction following bilateral nerve sparing radical retropubic prostatectomy: a randomized, double-blind, placebo controlled trial. Urology. 2004; 172 :1036–41. Erratum in: J Urol, 2005;173:664. [PubMed] [Google Scholar]
- NIH Consensus Development Panel on Impotence NIH Consensus Conference: impotence. JAMA. 1993; 270 :83–90. [PubMed] [Google Scholar]
- Padma-Nathan H, McCullough AR, Levine LA, et al. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res. 2008 Jul 24; [Epub ahead of print] [PubMed] [Google Scholar]
- Penson DF, Latini DM, Lubeck DP, et al. Do impotent men with diabetes have more severe erectile dysfunction and worse quality of life than the general population of impotent patients? Results from the Exploratory Comprehensive Evaluation of Erectile Dysfunction (ExCEED) database. Diabetes Care. 2003; 26 :1093–9. [PubMed] [Google Scholar]
- Penson DF, McLerran D, Feng Z, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the Prostate Cancer Outcomes Study. J Urol. 2008; 179 :S40–4. [PubMed] [Google Scholar]
- Porst H, Giuliano F, Glina S, et al. Evaluation of the efficacy and safety of once-a-day dosing of tadalafil 5 mg and 10 mg in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol. 2006; 50 :351–9. [PubMed] [Google Scholar]
- Porst H, Padma-Nathan H, Giuliano F, et al. Efficacy of tadalafil for the treatment of erectile dysfunction at 24 and 36 hours after dosing: a randomized controlled trial. Urology. 2003; 62 :121–5. [PubMed] [Google Scholar]
- Porst H, Rajfer J, Casabé A, et al. Long-term safety and efficacy of tadalafil 5 mg dosed once daily in men with erectile dysfunction. J Sex Med. 2008 Jun 13; [Epub ahead of print] [PubMed] [Google Scholar]
- Porst H, Rosen RC, Padma-Nathan H, et al. Tadalafil allows men with erectile dysfunction to have successful intercourse up to 36 hours postdose [abstract] J Urol. 2002; 167 :177. [Google Scholar]
- Raina R, Pahlajani G, Agarwal A, et al. Early penile rehabilitation following radical prostatectomy: Cleveland clinic experience. Int J Impot Res. 2008; 20 :121–6. [PubMed] [Google Scholar]
- Rajfer J, Aliotta PJ, Steidle CP, et al. Tadalafil dosed once a day in men with erectile dysfunction: a randomized, double-blind, placebo-controlled study in the US. Int J Impot Res. 2007; 19 :95–103. [PubMed] [Google Scholar]
- Rambhatla A, Kovanecz I, Ferrini M, et al. Rationale for phosphodiesterase 5 inhibitor use post-radical prostatectomy: experimental and clinical review. Int J Impot Res. 2008; 20 :30–4. [PubMed] [Google Scholar]
- Roehrborn CG, McVary KT, Elion-Mboussa A, et al. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J Urol. 2008; 180 :1228–34. [PubMed] [Google Scholar]
- Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997; 49 :822–30. [PubMed] [Google Scholar]
- Sáenz de Tejada I, Anglin G, Knight JR, et al. Effects of tadalafil on erectile dysfunction in men with diabetes. Diabetes Care. 2002; 25 :2159–64. [PubMed] [Google Scholar]
- Stanford JL, Feng J, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000; 283 :354–60. [PubMed] [Google Scholar]
- Ströberg P, Murphy A, Costigan T. Switching patients with erectile dysfunction from sildenafil citrate to tadalafil: results of a European multicenter, open-label study of patient preference. Clin Ther. 2003; 25 :2724–37. [PubMed] [Google Scholar]
- Sutherland EW, Rall TW. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem. 1958; 232 :1077–91. [PubMed] [Google Scholar]
- Talcott JA, Rieker P, Propert KJ, et al. Patient-reported impotence and incontinence after nerve-sparing radical prostatectomy. J Natl Cancer Inst. 1997; 89 :1117–23. [PubMed] [Google Scholar]
- Uckert S, Kuthe A, Jonas U, et al. Characterization and functional relevance of cyclic nucleotide phosphodiesterase isoenzymes of the human prostate. J Urol. 2001; 166 :2484–90. [PubMed] [Google Scholar]
- Vignozzi L, Filippi S, Morelli A, et al. Effect of chronic tadalafil administration on penile hypoxia induced by cavernous neurotomy in the rat. J Sex Med. 2006; 3 :419–31. [PubMed] [Google Scholar]
- von Keitz A, Rajfer J, Segal S, et al. A multicenter, randomized, double-blind, crossover study to evaluate patient preference between tadalafil and sildenafil. Eur Urol. 2004; 45 :499–507. [PubMed] [Google Scholar]
- Walsh PC, Lepor H, Eggleston JC. Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate. 1983; 4 :473–85. [PubMed] [Google Scholar]
- Walsh PC, Marschke P, Ricker D, et al. Patient-reported urinary continence and sexual function after anatomic radical prostatectomy. Urology. 2000; 55 :58–61. [PubMed] [Google Scholar]
7 Types of Erectile Dysfunction Treatments
There’s certainly no shortage of medications, supplements, and herbal remedies that claim to boost testosterone levels and sex drive. Especially if you have been recently experiencing performance issues in the bedroom, you might even be considering some of these alternatives.
But there’s a lot more to sexual health than just stamina or performance. If someone experiences difficulty having an erection, many of these products (despite their spectacular claims) are rarely effective, particularly when a more direct and scientifically researched treatment is needed to treat issues like erectile dysfunction.
Language matters
Sex and gender exist on spectrums. For the purposes of this article, we use “male” and “female” to refer to a person’s sex assigned at birth. Learn more about sex and gender.
Erectile dysfunction (ED) is when someone cannot achieve a firm erection or loses their erection before or during sexual activity.
Losing an erection can happen sometimes and shouldn’t be a major cause for concern every once in a while. But if it starts occurring several times a week and over a period of four or more weeks, it may be an indicator of ED.
Contrary to popular belief, ED isn’t a condition that just affects older males. Young males can experience ED too — and it’s actually more common than some people might realize. The Urology Care Foundation estimates that this condition affects approximately 30 million men in the United States.
Erectile dysfunction can develop as the result of several different factors, both physical and psychological, that contribute to a person’s ED symptoms. Talking with your doctor can help pinpoint what’s possibly causing your ED and you can both start to build an effective treatment plan.
For many people, making changes to their daily routine can help reduce ED symptoms. This could include diet modification, talk therapy, or erectile dysfunction medications.
Not everyone with ED needs to take medications. You should be aware of the cause of your ED before starting a medication.
If you think you have ED, see your primary care doctor. They’ll do a physical exam and request certain lab tests, as well as a complete medical and psychosocial history.
They may also refer you to a mental health professional who can help you manage performance anxiety or relationship issues related to your ED.
ED caused by underlying conditions
Your ED may be caused by untreated diabetes, high blood pressure, or another issue. Treating that condition first may improve your ED symptoms.
ED caused by medications
Other medications you’re taking may cause ED. These may include drugs used to treat:
Your doctor can also review any medications you’re currently taking and make some changes that could improve your ED.
Other causes of ED
There are other things that can contribute to ED. Adopting habits that promote overall health and well-being may help improve your symptoms.
Try limiting or avoiding smoking if you smoke, maintaining a moderate weight, getting regular exercise as often as possible, and limiting alcohol intake.
ED commonly occurs when blood flow to the penis is restricted or inhibited. When aroused, if blood is unable to flow to and from the penis effectively, it can’t stiffen into an erection or be kept for the entire length of a sexual encounter.
ED medications work by reversing or limiting the impact of whatever’s inhibiting that blood flow. One of the most common ED medications is known as a PDE5 inhibitor.
PDE5 stands for phosphodiesterase type 5 and it’s an enzyme that builds up over time and prevents regular blood flow to and from the penis. Taking a PDE5 inhibitor temporarily limits the effect of PDE5 and allows a person to more easily have an erection.
Less common treatments focus on improving heart health and blood pressure as a way to minimize ED symptoms.
Your doctor may want to explore other treatment options, especially if traditional PDE5 inhibitors aren’t working for you, because ED can be an early indicator for more severe health conditions like diabetes or heart disease.
If you’re looking for options to treat your ED, we have gathered this list of the most common ED medications and how effective they are at treating ED.
1. Alprostadil
Alprostadil (Caverject, Edex, MUSE) comes as an injectable solution and as a penile suppository.
You’ll inject the solution directly into your penis 5 to 20 minutes before having sex. You can use it as needed up to three times per week. You should let at least 24 hours lapse between injections.
With MUSE (or Medicated Urethral System for Erections), the suppository should be administered 5 to 10 minutes before sex. It should not be used more than twice in a 24-hour period.
Price for alprostadil
Currently, there’s no generic version of alprostadil available on the market, just the three brand name options. This means that the price for alprostadil will be higher when compared with generic alternatives to other ED medications.
According to GoodRX, the average retail price for two cartridges of 10mcg is around $180.
Pros
- studies have proven its effectiveness
- FDA-approved as an injectable treatment for ED
- use as needed and works within minutes
- alternative for people who didn’t respond well to oral pills
Cons
Common side effects of alprostadil
2. Avanafil
Avanafil (Stendra) is an oral drug and a PDE5 inhibitor. You should take it about 15 minutes before having sex. Do not take it more than once per day.
You should not use any PDE5 inhibitors if you’re also taking nitrates to treat heart disease. Examples of nitrates include isosorbide mononitrate (Monoket) and nitroglycerin (Nitrostat). Taking nitrates with avanafil can cause severely low blood pressure and even death.
Price for avanafil
Avanafil is currently only available as the brand name drug, Stendra. Like with most brand-name medications, the overall cost will likely be higher when compared with other generic alternatives.
The price for avanafil can vary widely based on the pharmacy filling the prescription. Retail prices generally range from $320 to $405 for six 200mg tablets, according to GoodRX.
Pros
- classified as a PDE5 inhibitor, one of the most common ED treatments
- FDA-approved medication
- effective in 15 to 30 minutes
- minimal side effects
Cons
Common side effects of avanafil
3. Sildenafil
Introduced in 1998 as the first PDE5 inhibitor, sildenafil (Viagra) is probably the most widely known ED treatment.
Viagra is only available as an oral tablet and there are also several generic versions of the drug available at a lower price. It should only be taken once per day, as needed. It can take about 30 minutes to start working and should be taken about an hour or so before sex to lower the chances of ED.
Price for sildenafil
Sildenafil can be a very budget-friendly erectile dysfunction medication and thanks to several online prescriber services like Roman and Hims, the price is very affordable even without insurance.
Generic sildenafil can cost as little as $2 per pill from some providers.
Pros
- as the first PDE5 inhibitor, several studies have proven its effectiveness, even from as far back as 2002
- FDA-approved medication
- only needs to be taken as needed
- very affordable price for generic sildenafil
Cons
- may have more minor side effects, like upset stomach or vision changes, compared to newer medications
- still requires a prescription, despite how long it has been on the market
Common side effects of sildenafil
- headache
- flushing
- stuffy or runny nose
- back pain
- upset stomach
- muscle aches
- vision changes, like blurry vision and changes in how certain colors look
4. Tadalafil
Tadalafil (Cialis) is an oral drug that increases blood flow throughout your body.
You can take this PDE5 inhibitor at a higher dose about 30 minutes before sex — no more than once per day — or at a lower dose every day so you’re always ready for sex and don’t need to plan ahead. It may work for up to 36 hours.
Price for tadalafil
Tadalafil is available as brand name Cialis or as generic tadalafil.
Depending on your needs, you may consider taking a higher dose that’ll be taken as needed but at a higher price per pill. Or, consider taking a lower dose every day, which costs less per pill, but the overall cost can add up quickly when buying a full month’s supply of pills.
As of September 2022, Generic daily 5mg tadalafil can cost around $8 per dose and higher doses of generic tadalafil — 10mg and 20mg — can start around $44 per pill.
Pros
- can be taken as needed at a higher dose or daily at a lower dose
- FDA-approved medication
- very affordable price for generic tadalafil
- can be effective for up to 36 hours at a higher dosage
Cons
- daily brand name Cialis can be too expensive for some budgets
- some users experience discomfort from specific side effects and explore other options
- requires a prescription, regardless of dosage
Common side effects of tadalafil
5. Testosterone
Testosterone is the primary sex hormone in the male body. It plays many roles in overall health.
Testosterone levels naturally drop with age. This change can lead to ED and other issues, like:
Doctors sometimes prescribe testosterone to treat ED. In fact, PDE5 inhibitors are most effective when used alongside testosterone therapy in people with a testosterone deficiency. But the drug does come with risks.
Testosterone can increase your chance of a heart attack or stroke. Because of these risks, the Food and Drug Administration (FDA) says that only people who have low testosterone due to certain health issues should use testosterone.
Your doctor will watch you closely if they prescribe testosterone. They’ll test the levels of testosterone in your body before and during your treatment with this drug. If your testosterone levels are too high, your doctor will stop your treatment or lower your dosage.
Price for testosterone
Testosterone therapy and testosterone treatment options can come in many forms, including:
As a result, it’s difficult to establish a price range that’s all-inclusive and doesn’t vary widely across all the different types. If you or your doctor are considering testosterone as a treatment for ED, it’s worth also discussing with them the overall cost to you and which treatments best fit into your budget.
Pros
- can have additional health benefits beyond ED treatment
- several different delivery methods to match your daily routine
- effective ED treatment when used alongside PDE5 inhibitors
Cons
- may require frequent testing of testosterone levels
- some types, like gels and creams, can be very potent and require very careful application
- side effects can be very extreme compared to other ED treatments
Possible side effects of testosterone
- acne
- male breasts
- prostate growth
- fluid retention that causes swelling
- moodiness
- sleep apnea, or interrupted breathing during your sleep
Testosterone for ED comes in many forms. The table below lists the forms of testosterone and their brand-name versions. Some forms may also be available as generic drugs.
| Testosterone form | Brand names |
|---|---|
| transdermal cream | First Testosterone Cream 2% |
| transdermal gel | AndroGel, Fortesta, Testim, and Vogelxo |
| transdermal patch | Androderm |
| transdermal solution | None (only available as generic) |
| topical gel | AndroGel and Natesto |
| nasal gel | Natesto |
| oral capsule | Testred |
| oral tablet | Android 25 |
| mucoadhesive film that dissolves under your gums | Striant |
| pellet implant | Testopel |
| solution for intramuscular injection | Depo-Testosterone and Aveed |
6. Vardenafil
Vardenafil (Levitra, Staxyn) is an oral drug and PDE5 inhibitor. It’s also available only with a prescription.
You should take it as needed about 60 minutes before sex but for some people, it can start working in about 30 minutes. You can take this drug up to once per day and it’s effective for about 5 to 7 hours.
Price for vardenafil
Depending on the pharmacy and even the state you’ll be buying it from, can cause the price for vardenafil to really vary. But generally speaking, the price can range from around $78 to $150 for ten 20mg tablets according to GoodRX.
Pros
- very popular because it’s one of the fastest-acting PDE5 inhibitors
- FDA-approved medication
- strong side effects are uncommon (the most common side effect is headache)
- available in generic form at a lower price than brand-name Levitra and Staxyn
Cons
Common side effects of vardenafil
7. Vitamins and supplements for ED
There are many vitamins and supplements on the market that claim to help ED. Some promise better sexual function as well as increased energy and vitality. But these supplements usually do not work. They may also be unsafe.
Some supplements that are marketed as “natural” may even contain drugs. ED supplements can still interact with other medications you’re taking. They may also cause side effects.
Talk with your doctor before trying any vitamins or supplements for ED.
The best place to get medications for ED is from your doctor or a licensed medical professional. Although some online pharmacies may sell or deliver ED medications, these are often unregulated and may contain ineffective or harmful ingredients.
ED medications vary in price. You can sign up for a subscription service, like Hims or Roman, and save money by purchasing multiple-month shipments.
If you’re getting these medications at a pharmacy with insurance, the cost will vary, too. Some coupons can be found online if you search for the medication you’re trying to get.
Without insurance or participating in one of these subscription services, the prices of ED medications — especially the name-brand versions — are quite expensive.
The average cost per pill of Viagra, for example, is $70. But sildenafil, the generic version of Viagra, costs as low as $9 per pill. Many services sell these therapies, so a bit of research should help you keep some money in your wallet.
How well do ED pills work?
The ED pills mentioned above have differences, including price points. Sildenafil has been on the market the longest, and its side effects are well known. It’s often the first erectile dysfunction medication that doctors turn to.
While medication for ED works often, sometimes addressing the underlying causes can be helpful too. Some ED drugs can have unpleasant side effects, like headaches, that cause discomfort.
It’s worth noting that some ED medications have different onset times in different people. The time these medications are effective also varies in individuals.
How quickly do ED pills work?
Most ED medications take about 15-45 minutes on average to work.
Sildenafil takes between 30 minutes and an hour to work, and it works for between 1 and 4 hours. Cialis can take up to 2 hours and last for 36 hours. There’s also a Cialis regimen that’s taken daily.
What are the side effects of ED pills?
Side effects of ED pills can range from headaches to nausea and diarrhea. Other mild side effects include warmness in the chest, neck or face, and a stuffy nose.
Some of the more serious side effects include back pain, loss of hearing, ringing in the ears, and the inability to see the differences between green and blue.
If you have underlying health conditions, you’ll want to abstain from ED meds or consult your physician for guidance.
Some of these conditions include liver disease, kidney disease, and low or uncontrolled high blood pressure. Nitrate drugs and ED medications should not be used at the same time because this combination could have serious health implications.
How do I choose the best medication for me?
These drugs are similar in their efficacy, so personal preference is important. Consider cost, ease of use, how long the drug lasts, and if you want a medication for one-time use or ongoing therapy.
There are generic versions of sildenafil, vardenafil, and tadalafil available, and you can get these medications via subscription services like Hims or Roman.
If you have signs of ED, keep in mind that the condition is often caused by another medication you’re taking or a health condition. Getting treatment for the underlying health issue or having your doctor adjust your drug regimen may be all that’s needed to ease your symptoms.
If you do need ED medications, there are many options. They come in different forms and work in unique ways. They also can cause side effects that are specific to the medication.
Together, you and your doctor can find the best ED medication for you.
ED is a common condition that can affect a person’s self-esteem and relationships. ED can be caused by underlying health issues or occur as a normal part of the aging process. Fortunately, there are many approved treatments to treat it.
There are a wide variety of ED meds, so choosing one based on cost and ease of use is usually the best place to start.
As always, if you have any health conditions or are on medications, be sure to speak with a physician first. These medications can have rare but sometimes serious, life threatening side effects.
Last medically reviewed on September 29, 2022
How we reviewed this article:
Healthline has strict sourcing guidelines and relies on peer-reviewed studies, academic research institutions, and medical associations. We avoid using tertiary references. You can learn more about how we ensure our content is accurate and current by reading our editorial policy.
- Adcirca – tadalafil tablet. (2020).
dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ff61b237-be8e-461b-8114-78c52a8ad0ae - Anaissie J, et al. (2016). Clinical use of alprostadil topical cream in patients with erectile dysfunction: A review.
ncbi.nlm.nih.gov/pmc/articles/PMC4977016/ - Burnett AL, et al. (2018). Erectile dysfunction: AUA guideline.
auajournals.org/doi/10.1016/j.juro.2018.05.004 - Carson CC, et al. (2002). The efficacy of sildenafil citrate (Viagra) in clinical populations: An update.
pubmed.ncbi.nlm.nih.gov/12414330/ - Cialis – tadalafil tablet, film coated. (2018).
dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bcd8f8ab-81a2-4891-83db-24a0b0e25895 - Erectile dysfunction. (n.d.).
niddk.nih.gov/health-information/urologic-diseases/erectile-dysfunction - Erectile dysfunction/sexual enhancement. (2017).
nccih.nih.gov/health/sex/erectiledysfunction.htm - FDA drug safety communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use. (2018).
fda.gov/Drugs/DrugSafety/ucm436259.htm - Huang SA, et al. (2013). Phosphodiesterase-5 (PDE5) inhibitors in the management of erectile dysfunction.
ncbi.nlm.nih.gov/pmc/articles/PMC3776492 - Jain A, et al. (2022). Alprostadil.
ncbi.nlm.nih.gov/books/NBK542217/ - Levitra – vardenafil hydrochloride tablet, film coated. (2018).
dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a01def95-c0ef-43b9-bd9e-5565b2385ad3 - Low testosterone (hypogonadism). (2020).
hormone.org/diseases-and-conditions/mens-health/low-testosterone - Muse – alprostadil suppository. (2018).
dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4c55f3f9-c4cf-11df-851a-0800200c9a66 - Papaverine hydrochloride – papaverine hydrochloride injection, solution. (2019).
dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9e5e2ce4-7a57-4c61-a826-64c8d11d038e - Sildenafil (including Viagra). (2019). https://www.nhs.uk/medicines/sildenafil-viagra/
- Staxyn – vardenafil hydrochloride tablet, orally disintegrating tablets. (2017).
dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0e5139d8-bf61-4f21-a36b-81b96b9b07d1 - Stendra – avanafil tablet. (2021).
dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a8726f90-9329-46ca-9379-2b50c78fe0e2 - Viagra – sildenafil citrate tablet, film coated. (2021).
dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0b0be196-0c62-461c-94f4-9a35339b4501 - What is erectile dysfunction? (2018).
urologyhealth.org/urologic-conditions/erectile-dysfunction
Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available.
Drug Interactions With Phosphodiesterase-5 Inhibitors Used for the Treatment of Erectile Dysfunction or Pulmonary Hypertension
From the Heart Institute, Good Samaritan Hospital (B.G.S., R.A.K.), and Department of Internal Medicine, Division of Cardiovascular Medicine, Keck School of Medicine at the University of Southern California (R.A.K.), Los Angeles.
From the Heart Institute, Good Samaritan Hospital (B.G.S., R.A.K.), and Department of Internal Medicine, Division of Cardiovascular Medicine, Keck School of Medicine at the University of Southern California (R.A.K.), Los Angeles.
Originally published 6 Jul 2010 https://doi.org/10.1161/CIRCULATIONAHA.110.944603 Circulation. 2010;122:88–95
Sildenafil and tadalafil were the 32nd and 74th, respectively, most popular prescription drugs dispensed in the United States in 2006. Erectile dysfunction (ED) currently affects >30 million men in the United States and >150 million men worldwide and will become more prevalent as the population ages. 1 Phosphodiesterase-5 (PDE5) inhibitors (PDE5Is) (sildenafil [Viagra], 2 vardenafil [Levitra], 3 and tadalafil [Cialis] 4 ) are first-line therapy for ED. Use of PDE5Is will increase because sildenafil (Revatio) 5 and tadalafil (Adcirca) 6 are now prescribed as first-line therapy for many patients with pulmonary hypertension (PHT). 5–8
Several pooled analyses comprising dozens of trials and thousands of patients, including patients with coronary artery disease and on antihypertensive medications, reported that PDE5Is did not significantly affect the incidence of adverse cardiovascular events. 9–12 However, PDE5 is distributed in many tissues, including platelets, veins, and arterial smooth muscle (pulmonary, coronary, and systemic arteries). 13 Thus, PDE5Is affect the cardiovascular system, mostly via vasodilation, and often cause small decreases in blood pressure (BP). When PDE5Is are coadministered with nitrates or α-blockers, pronounced systemic vasodilation and severe hypotension are possible. Many patients with ED are elderly and have the same risk factors as patients with coronary artery disease, so these drug combinations are commonly considered or encountered in clinical practice. 1 This article covers the important PDE5I drug interactions, including antihypertensive agents, nitrates, α-blockers, PHT agents, cytochrome P450 inhibitors, and other miscellaneous drugs.
Metabolic Clearance
Sildenafil is metabolized mainly by the cytochrome P450 3A4 pathway (79%) and to a lesser extent by 2C9 (20%). 14,15 Vardenafil is metabolized in a similar manner, mainly by 3A4 with a smaller contribution by 2C9. 15 Tadalafil is metabolized almost solely by 3A4. 15 Therefore, drugs that inhibit the 3A4 pathway decrease the metabolism and increase the plasma concentrations of PDE5Is (Table 1). Area under the concentration-time curve (exposure) values for sildenafil ranged from 0.8- to 2.6-fold (mean, 1.2-fold increase) when coadministered with 250 mL grapefruit juice (a 3A4 inhibitor), and results would vary even more in an uncontrolled setting (Table 1). 20 Data on changes in half-life and elimination time are less frequently reported, but 3A4 inhibitors reportedly have small effects or no effect on the half-lives of PDE5Is. 15 An exception is ritonavir, a potent inhibitor of several P450 cytochromes, including 3A4 and 2C9. By simultaneously blocking both pathways, ritonavir prevents a compensatory shift to the 2C9 pathway. Ritonavir increases exposure to sildenafil by 11-fold and increases the half-life of vardenafil from 4 to 26 hours. 3,18 Ritonavir initially inhibits 3A4 but later induces 3A4 after ≈1 week of steady-state levels. 6 The timing of observation likely accounts for the large difference in effects reported with ritonavir coadministration (Table 1). Cimetidine, a less potent, nonspecific cytochrome P450 inhibitor, increases exposure to sildenafil by 1.6-fold. 21 Ketoconazole inhibits sildenafil metabolism to a degree similar to ritonavir. 14 Although each drug combination has not been studied, the following 3A4 inhibitors would likely increase exposure to each of the PDE5Is: erythromycin, ketoconazole, itraconazole, clarithromycin, HIV protease inhibitors, and grapefruit juice. 2–4 The 2C9 inhibitors do not significantly affect the metabolism of PDE5Is. 15 It is unlikely and there is no evidence to date that other drugs metabolized by the 3A4 pathway competitively inhibit PDE5I metabolism because the 3A4 system has a high capacity. 2–4,15,23
Table 1. PDE5I Drug Interactions Involving Cytochrome P450 Isoenzyme CYP3A4
High doses of sildenafil (up to 800 mg) increased the incidence rates and severities of adverse events, although the types of adverse events were similar to those observed with lower doses, including visual disturbances, hypotension, syncope, and prolonged erection. 2 It is likely that higher plasma concentrations resulting from coadministration of 3A4 inhibitors would similarly influence the side-effect profile. For instance, the combination of itraconazole and tadalafil caused priapism in a healthy 56-year-old man. 24 In addition, higher PDE5I plasma concentrations resulting from 3A4 inhibitors can influence the severity and timing of other PDE5I drug interactions, including with nitrates and α-blockers. PDE5I dose adjustments are usually indicated when coadministered with 3A4 inhibitors (Table 2).
Table 2. Dosing of PDE5Is
Conversely, inducers of P450 3A4 increase the clearance and decrease the plasma concentrations of PDE5Is. 2,4 Rifampin reduced exposure to tadalafil by 88%. 4 Although each interaction has not been studied, other 3A4 inducers, including carbamazepine, phenytoin, and phenobarbital, would likely decrease PDE5I plasma levels. 2,4 No initial PDE5I dose adjustment is indicated when coadministered with 3A4 inducers; however, efficacy may be reduced in some patients requiring an increased dose. Adcirca is not recommended for patients taking long-term rifampin. 6
Notably, the metabolism of sildenafil was not affected by warfarin, azithromycin, 16 selective serotonin reuptake inhibitors, thiazides, angiotensin-converting enzyme inhibitors, calcium channel blockers, or antacid. 21 Vardenafil was not affected by warfarin, glyburide, digoxin, or ranitidine. Tadalafil was not affected by warfarin, midazolam, lovastatin, or theophylline. 2–4
Sildenafil and vardenafil weakly inhibit several cytochrome P450 pathways at doses much higher than recommended doses (ie, plasma concentrations 20 times higher than achieved with vardenafil 80 mg). 3,15 There is no evidence to date that sildenafil or vardenafil affects the clearance of other commonly used drugs, including warfarin, digoxin, atorvastatin, ritonavir, amlodipine, 25 and slow-release nifedipine. 2,3 Tadalafil does not inhibit or induce cytochrome P450 pathways and had no significant effect on the pharmacokinetics of digoxin, theophylline, warfarin, midazolam, or lovastatin. 4,15
Pharmacodynamics and Dosing
Sildenafil and vardenafil each have a half-life of 3 to 4 hours, lead to peak plasma levels ≈60 minutes after ingestion, and exhibit reduced absorption with a high-fat meal. 15 The half-life of sildenafil increases as the dose increases (25 mg, 2.6 hours; 100 mg, 3.7 hours). 26 Tadalafil has a half-life of 17.5 hours, which peaks at 2 hours, and its absorption is not influenced by food. 15 Tadalafil can be prescribed for ED as on-demand or once-daily dosing or as once daily for PHT. A dosing guide is provided in Table 2. Refer to the Physicians’ Desk Reference and its updates or product prescribing information for specific dose adjustments. 2–6 Higher plasma concentrations are desirable for the treatment of PHT compared with ED. Of note, some physicians prescribe higher doses of sildenafil (up to 80 mg 3 TID) for PHT than recommended (20 mg TID) on the basis of the doses used in a few clinical trials of PHT. 27,28 Although comparisons were made only with placebo, sildenafil 80 mg TID appeared to improve World Health Organization functional class more than 20 mg TID (placebo-corrected proportion of patients with improvement of at least 1 functional class with sildenafil 80 mg TID, 42%; with 20 mg TID, 28%). 28
Antihypertensives and PDE5Is
PDE5Is can be coadministered with most antihypertensive medications without inducing clinically significant reductions in BP. 29–31 Caution must be used with α-blockers. In general, the BP reductions caused by PDE5Is are small whether they are taken alone or in conjunction with other antihypertensive medications, including β-blockers, diuretics, calcium channel blockers, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers (Table 3). In most healthy subjects, BP returned to baseline values within 6 hours of sildenafil administration. 34 After tadalafil administration, diastolic BP decreased slightly and remained low for 12 hours, whereas systolic BP (SBP) did not change. 33 Coadministration of sildenafil with antihypertensive medications was evaluated in a posthoc subanalysis of 18 trials including 3975 men, 1094 of whom were also taking at least 1 antihypertensive medication. 30 The incidence of adverse events and adverse events potentially related to BP was similar between men with and those without antihypertensive medications (34% versus 38%) and was also similar in men with multiple antihypertensive medications and between each individual medication. Even coadministration of multiple antihypertensive medications with the relatively longer-acting tadalafil did not increase the occurrence of potentially clinically significant decreases in BP. 29 PDE5Is precipitate little or no change in heart rate. 12,25,33,34,36 Although PDE5Is are generally safe with most vasodilating medications, the exceptions are nitrates and α-blockers, described next.
Table 3. Interactions Between PDE5Is and Various Antihypertensive Medications
Nitrates and PDE5Is
Penile erections and endothelium-mediated vasodilation are mediated through cGMP, which promotes trabecular and vascular smooth muscle relaxation. PDE5Is prevent the breakdown of cGMP. Nitric oxide donors (ie, nitrates) increase the production of cGMP. Because both PDE5Is and nitrates increase cGMP, coadministration can generate excess accumulation of cGMP and can trigger marked vasodilation and severe hypotension. For instance, when Emmick and colleagues 37 analyzed nitroglycerin 0.4 mg in combination with sildenafil 50 mg, tadalafil 10 mg, and placebo, a potentially clinically significant change in BP was observed more frequently with each PDE5I than with placebo (standing SBP
Duration of Contraindication
If a patient has taken a PDE5I and then develops stable angina, unstable angina, or a myocardial infarction, when can nitrates be administered safely? The American College of Cardiology and American Heart Association suggest nitrates can be administered 24 hours (6 half-lives) after sildenafil intake to allow full clearance of the drug. 23 The study by Emmick and colleagues 37 evaluated nitroglycerin administration 1 day after sildenafil, tadalafil, or placebo. The incidence of a significant decrease in BP was similar between sildenafil and placebo but inconclusive for tadalafil (standing SBP 30 mm Hg: placebo, 12%; sildenafil, 4%; tadalafil, 20%). Sildenafil no longer showed evidence of an interaction with nitroglycerin 24 hours after sildenafil administration. 37 The interaction with nitrates may be gone as early as 4 hours after sildenafil intake. When nitroglycerin was administered 4 hours after sildenafil or placebo, there was no significant difference in mean maximal change from baseline BP; however, this abstract did not report the incidence of potentially clinically significant decreases in BP. 38 Likewise, vardenafil is suggested to lack an interaction with nitrates at 24 hours after intake (6 half-lives). 3 Conversely, tadalafil does interact with sublingual nitroglycerin to increase the risk of hypotension at 24 hours but not at 48 hours after tadalafil intake. 39 After receiving tadalafil 20 mg or placebo for 7 consecutive days, 150 men were given repeated doses of nitroglycerin 0.4 mg. At 4, 8, and 24 hours after the last tadalafil intake, nitroglycerin caused more subjects in the tadalafil group than in the placebo group to experience a potentially clinically significant decrease in BP, including a standing SBP
Treatment of PDE5I-Nitrate–Induced Hypotension
What if a patient has taken a PDE5I, receives a nitrate, and becomes hypotensive from pronounced vasodilation? The American College of Cardiology and AHA suggest placing the patient in the Trendelenburg position, aggressive fluid resuscitation, and if necessary an α-agonist (phenylephrine), a β-agonist (norepinephrine), and intraaortic balloon counterpulsation. 23 There is no antidote to PDE5Is.
α-Blockers and PDE5Is
“Uroselective” α-blockers (tamsulosin, alfuzosin) preferentially inhibit α1A and α1D receptors found primarily in the prostate and benefit patients with benign prostatic hypertrophy. Other α-blockers (terazosin) are less selective, and some (doxazosin) are used as third-line agents for hypertension because of their higher affinity for α1B receptors, which are abundant in the peripheral vasculature. 40–42 All α-blockers can cause vasodilation and orthostatic hypotension, and coadministration with PDE5Is increases the risk of a clinically significant decrease in BP. Various combinations of PDE5Is and α-blockers interact to different degrees, as shown in Table 4. The degree of PDE5I–α-blocker interaction depends on which drugs are coadministered, the dose of α-blocker, the timing of administration, and the duration or stability of the α-blocker therapy (Table 4). 42 Tadalafil has fewer effects on the cardiovascular system than the other PDE5Is, as exemplified by its minimal effects on BP in healthy control subjects (Table 3). 33
Table 4. Interactions Between PDE5Is and α-Blockers
Timing of Administration
Vardenafil was studied with terazosin and (in a separate study) tamsulosin, both simultaneously and separated by 6 hours (Table 4). 3 A standing SBP 30-mm Hg decrease in standing SBP occurred in 9 of 24 men receiving tamsulosin and in 19 of 29 men receiving terazosin and led to the early termination of the simultaneous administration of terazosin arm. Compared with administration 6 hours apart, simultaneous administration of vardenafil and terazosin more frequently resulted in a standing SBP 30-mm Hg decrease in standing SBP.
Uroselective α-Blockers
Coadministration of tadalafil with doxazosin and with tamsulosin was evaluated (Table 4). 43 Tamsulosin with tadalafil decreased SBP only minimally and did not increase the risk of a potentially clinically significant decrease in BP relative to placebo. Compared with doxazosin and placebo, doxazosin and tadalafil significantly reduced standing SBP (−9.8 mm Hg) and increased the incidence of a standing SBP
When sildenafil 100 mg alone was compared with sildenafil 100 mg and tamsulosin 0.4 mg coadministration, BP was not statistically different between groups in supine patients or after tilt testing. 45
When coadministration of tadalafil and alfuzosin (uroselective) was evaluated, the change in standing SBP was not statistically significant from tadalafil and placebo (Table 4). 41 Although 1 asymptomatic man had a standing SBP of 83 mm Hg, no man had a supine SBP 30-mm Hg decrease in SBP, or a diastolic BP
Stability of α-Blocker Therapy
Twenty-two men with benign prostatic hypertrophy on stable tamsulosin therapy for >4 weeks were given vardenafil or placebo (Table 4). 44 Small decreases in BP were observed, and the number of potentially clinically significant decreases in SBP was similar between vardenafil and placebo (1 versus 0). Of note, the study described in Timing of Administration did not specify the duration of tamsulosin therapy before vardenafil coadministration.
Dose of α-Blocker
Forty-five men were given tadalafil (5 mg) or placebo for 28 days, and beginning on day 8, increasing doses of doxazosin (1, 2, and 4 mg/d) were administered. 40 The total number of subjects experiencing a potentially clinically significant decrease in SBP or diastolic BP increased on the first day that doxazosin 4 mg was administered (9 of 39 with tadalafil and 7 of 40 with placebo) but decreased by the seventh day of doxazosin 4 mg coadministration (1 of 39 with tadalafil and 2 of 40 with placebo). Therefore, coadministration of doxazosin with long-term tadalafil appeared to have similar effects on BP as placebo; increasing the dose of doxazosin increased the incidence of potentially clinically significant decreases in BP on the first day of the 4 mg dose.
Collectively, these studies indicate that combining PDE5Is with α-blockers increases the risk of a clinically significant decrease in BP. This risk is reduced with tadalafil, with uroselective α-blockers, when low doses of α-blockers are used, when dosing is separated by several hours (instead of simultaneously), and when patients are on stable therapy with 1 agent before the other drug class is administered. 42 Consequently, for patients prescribed α-blockers, current Food and Drug Administration labeling states that PDE5Is are recommended only once α-blocker therapy has become stable. Once stability is achieved with an α-blocker, a PDE5I can be initiated at a low dose (Table 2). When starting α-blocker therapy for patients already optimized on a PDE5I, physicians should begin with the lowest α-blocker dose. Thereafter, increasing the dose of either the α-blocker or PDE5I may further lower BP.
Drugs for PHT and PDE5Is
Because sildenafil and tadalafil have been approved for PHT therapy and their use in combination therapy has been endorsed for certain World Health Organization class IV patients, 7 coadministration with other PHT agents warrants investigation. Administering sildenafil to patients with PHT already taking epoprostenol, 27 iloprost, 46,47 nitric oxide, 48,49 or bosentan 50,51 further improves an array of hemodynamic, clinical status, and exercise capacity parameters with little or no effect on systemic BP and without increasing adverse events or hypotension. Moreover, sildenafil prolonged the effect of inhaled nitric oxide on pulmonary vasodilatation and prevented rebound pulmonary vasoconstriction after inhaled nitric oxide. 49 Coadministration of epoprostenol reduced plasma concentrations of sildenafil by ≈25%, but this interaction was not considered clinically relevant. 5
Bosentan, a P450 3A4 inducer, reduced tadalafil exposure by 42%, whereas bosentan levels were unchanged. 6 Coadministration of bosentan and sildenafil reduced sildenafil exposure by 63% and increased bosentan exposure by 1.5-fold. 22 Coadministration with sildenafil does not increase the risk of liver aminotransferase elevation associated with bosentan. 52 A study of 405 patients with PHT (53% also receiving background bosentan therapy) showed that tadalafil improved 6-minute walk distance, time to clinical worsening, and the incidence of clinical worsening. 53 Interestingly, the increase in 6-minute walk distance was significant in bosentan-naïve patients (44 m; P<0.01) but not for patients on background bosentan (23 m; P=0.09). 53 The greater improvement for bosentan-naïve patients may support the ceiling phenomenon hypothesis that limits additional improvements in patients on background PHT therapy or may reflect the decrease in plasma concentration of PDE5I observed with bosentan coadministration. 53 Additional studies are underway to guide combination therapy.
In subjects given very high doses (sildenafil 800 mg), the types of adverse events were the same, but the incidences and severities of adverse events were increased. 2 Moreover, compared with the doses used for ED, 2,4 the higher doses used for PHT increased the incidences of adverse events. 5,6 For example, the incidence of headache with PHT doses (sildenafil, 46% 5 ; tadalafil, 42% 6 ) was higher compared with ED doses (sildenafil, 16% 2 ; tadalafil, 11% 4 ). Coadministration with 3A4 inhibitors would likely further increase the incidences of adverse events.
There is little direct evidence on the drug interactions of nitrates and α-blockers with the higher PHT doses of PDE5Is. The higher doses increase the plasma concentrations and prolong the elimination time of sildenafil. 4,26 Consequently, the contraindication with nitrates may extend beyond the previously discussed time frames derived from studies using ED doses. Conversely, combination therapy with PDE5Is and systemic nitrates could be therapeutic if the synergistic effect on cGMP and vasodilation remains relatively selective for the pulmonary vasculature as observed with PDE5Is alone. 54
The α-blocker data are inconclusive. In studies using different ED doses of PDE5Is with α-blockers, greater reductions in mean BP parameters and more frequent, potentially clinically significant decreases in BP were observed with higher doses, 4 but many reports were inconclusive, 2,3,43,44 and greater effects were reported with lower doses in some studies. 42
PDE5Is and Risk of Bleeding
The use of PDE5Is has not been evaluated in patients with bleeding disorders, with active peptic ulceration, or on multiple blood-thinning and antiplatelet agents. 2–4 PDE5Is may affect bleeding by a direct action on platelets, which contain PDE5. 55 The product inserts report that PDE5Is, alone or with aspirin, did not affect bleeding time. 2–4,6 Berkels and colleagues 55 reported that sildenafil 100 mg transiently prolonged bleeding time 1 hour after administration and that sildenafil 50 mg did not alter bleeding time. Compared with placebo, sildenafil increased the incidence of epistaxis in patients on concomitant vitamin K antagonists (9% versus 2%) and in patients with PHT secondary to connective tissue disease (13% versus 2%) but not in patients with primary PHT (3% versus 2%). 5 The incidence of epistaxis in PHT clinical trials was higher with sildenafil 27,28 than with placebo and minimally higher with tadalafil 53 compared with placebo. Epistaxis has been associated with on-demand use of sildenafil 56,57 and tadalafil 56,57 for enhanced sexual activity. Sildenafil inhibits ADP-dependent platelet aggregation in vitro, with an additive effect when combined with nitrates. 5,55 Sildenafil combined with heparin had an additive effect on bleeding time in rabbits but has not been studied in humans. 2 Theoretically, dipyridamole, ticlopidine, and clopidogrel may interact with PDE5Is, although no studies have been conducted to evaluate a potential interaction. In conclusion, PDE5Is appear to minimally increase the risk of minor bleeding especially when combined with vitamin K antagonists or nitrates.
Miscellaneous
Unlike other β-blockers, rather than impair sexual activity, nebivolol improves erectile function. 58,59 Nebivolol decreases plasma sildenafil concentration by ≈20%. 60 Coadministration with sildenafil does not potentiate the vasodilatory effect of nebivolol, 61 and the product insert for nebivolol indicates that the effect of the drug combination on pulse and BP is additive. 60
The tadalafil label cautions against ≥5 U alcohol (1 U is ≈1 oz of 80-proof liquor). 4,6 Orthostatic hypotension was observed in patients given tadalafil and 6 U alcohol but not with 4 U. 4 Sildenafil and vardenafil do not carry this warning.
Patient Education
Because of the potential for life-threatening hypotension, patients should be counseled appropriately on the drug interactions of PDE5Is. Patients should be warned to avoid all nitrates, including recreationally inhaled poppers and nitroglycerin from friends or family. Patients should be cautioned appropriately on α-blockers, cimetidine (which can be obtained over the counter), and grapefruit juice. If patients develop chest pain while on PDE5Is, it is crucial for them to divulge the use of PDE5Is to their healthcare providers. Patients should be instructed to contact emergency services if they experience severe dizziness, headache, or syncope that may be related to PDE5Is, and they should inform healthcare workers of their most recent PDE5I intake so that appropriate care can be given. Patients should not share PDE5Is with friends, family, or the “black market.”
Conclusions
PDE5Is are commonly prescribed and have benefited millions of men with ED and increasing numbers of patients with PHT. Although PDE5Is are safe with most antihypertensive agents, coadministration with nitrates or α-blockers poses a risk of severe hypotension. Nitrates are contraindicated within 24 hours of sildenafil and vardenafil and within 48 hours of tadalafil. Only after patients are on stable α-blocker therapy should PDE5Is be initiated, starting with a low dose. Metabolic interactions with bosentan may call for dose adjustments when combination therapy is used. Potent cytochrome P450 3A4 inhibitors, including erythromycin, clarithromycin, ketoconazole, itraconazole, and HIV protease inhibitors, increase PDE5I plasma concentrations. PDE5I drug interactions have the potential to cause life-threatening hypotension in patients with coexisting cardiac disease requiring nitrates or α blockers. Knowledge of these potential drug interactions is needed to avoid severe side effects.
Dr Kloner has served as a speaker and consultant to Pfizer and Lilly. Dr Schwartz reports no conflicts.
Footnotes
Reprint requests to Robert A. Kloner, MD, PhD, Heart Institute, Good Samaritan Hospital, 1225 Wilshire Blvd, Los Angeles, CA 90017-2395. E-mail [email protected]
References
- 1 Seftel AD, Sun P, Swindle R. The prevalence of hypertension, hyperlipidemia, diabetes mellitus and depression in men with erectile dysfunction. J Urol. 2004 ; 171: 2341–2345. CrossrefMedlineGoogle Scholar
- 2 Pfizer. VIAGRA (sildenafil citrate) prescribing information. Available at: http://www.pfizer.com/files/products/uspi_viagra.pdf. Accessed March 29, 2010. Google Scholar
- 3 Bayer Health Care Pharmaceuticals. Product monograph for LEVITRA (vardenafil HCL). Available at: http://www.univgraph.com/bayer/inserts/levitra.pdf. Accessed March 29, 2010. Google Scholar
- 4 Eli Lilly and Co. CIALIS (tadalafil) prescribing information. Available at: http://pi.lilly.com/us/cialis-pi.pdf. Accessed March 29, 2010. Google Scholar
- 5 Pfizer. REVATIO (sildenafil) prescribing information. Available at: http://media.pfizer.com/files/products/uspi_revatio.pdf. Accessed March 29, 2010. Google Scholar
- 6 Eli Lilly and Co. ADCIRCA (tadalafil) prescribing information. Available at: http://pi.lilly.com/us/adcirca-pi.pdf. Accessed March 29, 2010. Google Scholar
- 7 Barst RJ, Gibbs JS, Ghofrani HA, Hoeper MM, McLaughlin VV, Rubin LJ, Sitbon O, Tapson VF, Galie N. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol. 2009 ; 54: S78–S84. CrossrefMedlineGoogle Scholar
- 8 Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007 ; 131: 1917–1928. CrossrefMedlineGoogle Scholar
- 9 Mittleman MA, Glasser DB, Orazem J. Clinical trials of sildenafil citrate (Viagra) demonstrate no increase in risk of myocardial infarction and cardiovascular death compared with placebo. Int J Clin Pract. 2003 ; 57: 597–600. MedlineGoogle Scholar
- 10 Morales A, Gingell C, Collins M, Wicker PA, Osterloh IH. Clinical safety of oral sildenafil citrate (VIAGRA) in the treatment of erectile dysfunction. Int J Impot Res. 1998 ; 10: 69–73. CrossrefMedlineGoogle Scholar
- 11 Kloner RA, Jackson G, Hutter AM, Mittleman MA, Chan M, Warner MR, Costigan TM, Vail GM. Cardiovascular safety update of Tadalafil: retrospective analysis of data from placebo-controlled and open-label clinical trials of Tadalafil with as needed, three times-per-week or once-a-day dosing. Am J Cardiol. 2006 ; 97: 1778–1784. CrossrefMedlineGoogle Scholar
- 12 Kloner RA, Mohan P, Segeron T, Thibonnier M, Norenberg C, Padma-Nathan H. Cardiovascular safety of vardenafil in patients receiving antihypertensive medications: a post-hoc analysis of five placebo-controlled clinical trials. J Am Coll Cardiol. 2003 ; 41: 276A–277A. Abstract. Google Scholar
- 13 Wallis RM, Corbin JD, Francis SH, Ellis P. Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am J Cardiol. 1999 ; 83: 3C–12C. CrossrefMedlineGoogle Scholar
- 14 Warrington JS, Shader RI, von Moltke LL, Greenblatt DJ. In vitro biotransformation of sildenafil (Viagra): identification of human cytochromes and potential drug interactions. Drug Metab Dispos. 2000 ; 28: 392–397. MedlineGoogle Scholar
- 15 Gupta M, Kovar A, Meibohm B. The clinical pharmacokinetics of phosphodiesterase-5 inhibitors for erectile dysfunction. J Clin Pharmacol. 2005 ; 45: 987–1003. CrossrefMedlineGoogle Scholar
- 16 Muirhead GJ, Faulkner S, Harness JA, Taubel J. The effects of steady-state erythromycin and azithromycin on the pharmacokinetics of sildenafil in healthy volunteers. Br J Clin Pharmacol. 2002 ; 53: 37S–43S. CrossrefMedlineGoogle Scholar
- 17 Hedaya MA, El-Afify DR, El-Maghraby GM. The effect of ciprofloxacin and clarithromycin on sildenafil oral bioavailability in human volunteers. Biopharm Drug Dispos. 2006 ; 27: 103–110. CrossrefMedlineGoogle Scholar
- 18 Muirhead GJ, Wulff MB, Fielding A, Kleinermans D, Buss N. Pharmacokinetic interactions between sildenafil and saquinavir/ritonavir. Br J Clin Pharmacol. 2000 ; 50: 99–107. CrossrefMedlineGoogle Scholar
- 19 Christ B, Brockmeier D, Hauck EW, Friemann S. Interactions of sildenafil and tacrolimus in men with erectile dysfunction after kidney transplantation. Urology. 2001 ; 58: 589–593. CrossrefMedlineGoogle Scholar
- 20 Bailey DG, Dresser GK. Interactions between grapefruit juice and cardiovascular drugs. Am J Cardiovasc Drugs. 2004 ; 4: 281–297. CrossrefMedlineGoogle Scholar
- 21 Wilner K, Laboy L, LeBel M. The effects of cimetidine and antacid on the pharmacokinetic profile of sildenafil citrate in healthy male volunteers. Br J Clin Pharmacol. 2002 ; 53: 31S–36S. CrossrefMedlineGoogle Scholar
- 22 Burgess G, Hoogkamer H, Collings L, Dingemanse J. Mutual pharmacokinetic interactions between steady-state bosentan and sildenafil. Eur J Clin Pharmacol. 2008 ; 64: 43–50. CrossrefMedlineGoogle Scholar
- 23 Cheitlin MD, Hutter AM Jr, Brindis RG, Ganz P, Kaul S, Russell RO Jr, Zusman RM. Use of sildenafil (Viagra) in patients with cardiovascular disease: Technology and Practice Executive Committee. Circulation. 1999 ; 99: 168–177. CrossrefMedlineGoogle Scholar
- 24 Galatti L, Fioravanti A, Salvo F, Polimeni G, Giustini SE. Interaction between tadalafil and itraconazole. Ann Pharmacother. 2005 ; 39: 200. Google Scholar
- 25 Webb DJ, Freestone S, Allen MJ, Muirhead GJ. Sildenafil citrate and blood-pressure-lowering drugs: results of drug interaction studies with an organic nitrate and a calcium antagonist. Am J Cardiol. 1999 ; 83: 21C–28C. CrossrefMedlineGoogle Scholar
- 26 Nichols DJ, Muirhead GJ, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol. 2002 ; 53: 5S–12S. CrossrefMedlineGoogle Scholar
- 27 Simonneau G, Rubin LJ, Galie N, Barst RJ, Fleming TR, Frost AE, Engel PJ, Kramer MR, Burgess G, Collings L, Cossons N, Sitbon O, Badesch DB. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med. 2008 ; 149: 521–530. CrossrefMedlineGoogle Scholar
- 28 Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005 ; 353: 2148–2157. CrossrefMedlineGoogle Scholar
- 29 Kloner RA, Mitchell M, Emmick JT. Cardiovascular effects of tadalafil in patients on common antihypertensive therapies. Am J Cardiol. 2003 ; 92: 47M–57M. CrossrefMedlineGoogle Scholar
- 30 Kloner RA, Brown M, Prisant LM, Collins M. Effect of sildenafil in patients with erectile dysfunction taking antihypertensive therapy: Sildenafil Study Group. Am J Hypertens. 2001 ; 14: 70–73. CrossrefMedlineGoogle Scholar
- 31 Kloner RA, Sadovsky R, Johnson EG, Mo D, Ahuja S. Efficacy of tadalafil in the treatment of erectile dysfunction in hypertensive men on concomitant thiazide diuretic therapy. Int J Impot Res. 2005 ; 17: 450–454. CrossrefMedlineGoogle Scholar
- 32 Zusman RM, Morales A, Glasser DB, Osterloh IH. Overall cardiovascular profile of sildenafil citrate. Am J Cardiol. 1999 ; 83: 35C–44C. CrossrefMedlineGoogle Scholar
- 33 Kloner RA, Mitchell M, Emmick JT. Cardiovascular effects of tadalafil. Am J Cardiol. 2003 ; 92: 37M–46M. CrossrefMedlineGoogle Scholar
- 34 Jackson G, Benjamin N, Jackson N, Allen MJ. Effects of sildenafil citrate on human hemodynamics. Am J Cardiol. 1999 ; 83: 13C–20C. CrossrefMedlineGoogle Scholar
- 35 Rohde G, Jordan P. Influence of vardenafil on blood pressure and pharmacokinetics in hypertensive patients on nifedipine therapy. Pharmacotherapy. 2002 ; 22: 418. Abstract. Google Scholar
- 36 Zusman RM, Prisant LM, Brown MJ. Effect of sildenafil citrate on blood pressure and heart rate in men with erectile dysfunction taking concomitant antihypertensive medication: Sildenafil Study Group. J Hypertens. 2000 ; 18: 1865–1869. CrossrefMedlineGoogle Scholar
- 37 Emmick JT, Stuewe SR, Mitchell M. Overview of the cardiovascular effects of tadalafil. Eur Heart J Suppl. 2002 ; 4: H32–H47. CrossrefGoogle Scholar
- 38 Crowley AR, Muirhead GJ, Gillies H, Osterloh I. Interaction between glyceryl trinitrate and sildenafil citrate (Viagra®) may last less than 4 hours. Am J Hypertens. 2004 ; 17: S37. Abstract. CrossrefMedlineGoogle Scholar
- 39 Kloner RA, Hutter AM, Emmick JT, Mitchell MI, Denne J, Jackson G. Time course of the interaction between tadalafil and nitrates. J Am Coll Cardiol. 2003 ; 42: 1855–1860. CrossrefMedlineGoogle Scholar
- 40 Guillaume M, Lonsdale F, Darstein C, Jimenez MC, Mitchell MI. Hemodynamic interaction between a daily dosed phosphodiesterase 5 inhibitor, tadalafil, and the alpha-adrenergic blockers, doxazosin and tamsulosin, in middle-aged healthy male subjects. J Clin Pharmacol. 2007 ; 47: 1303–1310. CrossrefMedlineGoogle Scholar
- 41 Giuliano F, Kaplan SA, Cabanis MJ, Astruc B. Hemodynamic interaction study between the alpha1-blocker alfuzosin and the phosphodiesterase-5 inhibitor tadalafil in middle-aged healthy male subjects. Urology. 2006 ; 67: 1199–1204. CrossrefMedlineGoogle Scholar
- 42 Kloner RA. Pharmacology and drug interaction effects of the phosphodiesterase 5 inhibitors: focus on alpha-blocker interactions. Am J Cardiol. 2005 ; 96: 42M–46M. MedlineGoogle Scholar
- 43 Kloner RA, Jackson G, Emmick JT, Mitchell MI, Bedding A, Warner MR, Pereira A. Interaction between the phosphodiesterase 5 inhibitor, tadalafil and 2 alpha-blockers, doxazosin and tamsulosin in healthy normotensive men. J Urol. 2004 ; 172: 1935–1940. CrossrefMedlineGoogle Scholar
- 44 Auerback S, Gittelman M, Mazzu A, Sundaresan PR, White WB. Coadministered vardenafil (for erectile dysfunction) and tamsulosin do not induce hypotension in patients with benign prostatic hypertrophy. Am J Hypertens. 2004 ; 17: 16A. Abstract. MedlineGoogle Scholar
- 45 Nieminen T, Tammela TL, Koobi T, Kahonen M. The effects of tamsulosin and sildenafil in separate and combined regimens on detailed hemodynamics in patients with benign prostatic enlargement. J Urol. 2006 ; 176: 2551–2556. CrossrefMedlineGoogle Scholar
- 46 Wilkens H, Guth A, Konig J, Forestier N, Cremers B, Hennen B, Bohm M, Sybrecht GW. Effect of inhaled iloprost plus oral sildenafil in patients with primary pulmonary hypertension. Circulation. 2001 ; 104: 1218–1222. CrossrefMedlineGoogle Scholar
- 47 Ghofrani HA, Rose F, Schermuly RT, Olschewski H, Wiedemann R, Kreckel A, Weissmann N, Ghofrani S, Enke B, Seeger W, Grimminger F. Oral sildenafil as long-term adjunct therapy to inhaled iloprost in severe pulmonary arterial hypertension. J Am Coll Cardiol. 2003 ; 42: 158–164. CrossrefMedlineGoogle Scholar
- 48 Michelakis E, Tymchak W, Lien D, Webster L, Hashimoto K, Archer S. Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxide. Circulation. 2002 ; 105: 2398–2403. LinkGoogle Scholar
- 49 Lepore JJ, Maroo A, Pereira NL, Ginns LC, Dec GW, Zapol WM, Bloch KD, Semigran MJ. Effect of sildenafil on the acute pulmonary vasodilator response to inhaled nitric oxide in adults with primary pulmonary hypertension. Am J Cardiol. 2002 ; 90: 677–680. CrossrefMedlineGoogle Scholar
- 50 Gruenig E, Michelakis E, Vachiery JL, Vizza CD, Meyer FJ, Doelberg M, Bach D, Dingemanse J, Galie N. Acute hemodynamic effects of single-dose sildenafil when added to established bosentan therapy in patients with pulmonary arterial hypertension: results of the COMPASS-1 study. J Clin Pharmacol. 2009 ; 49: 1343–1352. CrossrefMedlineGoogle Scholar
- 51 Hoeper MM, Faulenbach C, Golpon H, Winkler J, Welte T, Niedermeyer J. Combination therapy with bosentan and sildenafil in idiopathic pulmonary arterial hypertension. Eur Respir J. 2004 ; 24: 1007–1010. CrossrefMedlineGoogle Scholar
- 52 Humbert M, Segal ES, Kiely DG, Carlsen J, Schwierin B, Hoeper MM. Results of European post-marketing surveillance of bosentan in pulmonary hypertension. Eur Respir J. 2007 ; 30: 338–344. CrossrefMedlineGoogle Scholar
- 53 Galie N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth A, Frumkin L, Barst RJ. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009 ; 119: 2894–2903. LinkGoogle Scholar
- 54 Stehlik J, Movsesian MA. Combined use of PDE5 inhibitors and nitrates in the treatment of pulmonary arterial hypertension in patients with heart failure. J Card Fail. 2009 ; 15: 31–34. CrossrefMedlineGoogle Scholar
- 55 Berkels R, Klotz T, Sticht G, Englemann U, Klaus W. Modulation of human platelet aggregation by the phosphodiesterase type 5 inhibitor sildenafil. J Cardiovasc Pharmacol. 2001 ; 37: 413–421. CrossrefMedlineGoogle Scholar
- 56 Pomara G, Morelli G, Menchini-Fabris F, Dinelli N, Campo G, LiGuori G, Selli C. Epistaxis after PDE-5 inhibitors misuse. Int J Impot Res. 2006 ; 18: 213–214. CrossrefMedlineGoogle Scholar
- 57 Ismail H, Harries PG. Recurrent epistaxis after treatment with tadalafil (Cialis). Acta Otolaryngol. 2005 ; 125: 334–335. CrossrefMedlineGoogle Scholar
- 58 Brixius K, Middeke M, Lichtenthal A, Jahn E, Schwinger RH. Nitric oxide, erectile dysfunction and beta-blocker treatment (MR NOED study): benefit of nebivolol versus metoprolol in hypertensive men. Clin Exp Pharmacol Physiol. 2007 ; 34: 327–331. CrossrefMedlineGoogle Scholar
- 59 Cordero A, Bertomeu-Martinez V, Mazon P, Facila L, Bertomeu-Gonzalez V, Conthe P, Gonzalez-Juanatey JR. Erectile dysfunction in high-risk hypertensive patients treated with beta-blockade agents. Cardiovasc Ther. 2010 ; 28: 15–22. CrossrefMedlineGoogle Scholar
- 60 Forest Laboratories, Inc. BYSTOLIC (nebivolol) prescribing information. Available at: http://www.frx.com/pi/Bystolic_pi.pdf. Accessed March 29, 2010. Google Scholar
- 61 Rosenkranz S, Brixius K, Halbach R, Diedrichs H, Schwinger RH. Phosphodiesterase type 5 inhibitor sildenafil citrate does not potentiate the vasodilative properties of nebivolol in rat aorta. Life Sci. 2006 ; 78: 1103–1107. CrossrefMedlineGoogle Scholar
- 62 Beasley CM Jr, Mitchell MI, Dmitrienko AA, Emmick JT, Shen W, Costigan TM, Bedding AW, Turik MA, Bakhtyari A, Warner MR, Ruskin JN, Cantilena LR Jr, Kloner RA. The combined use of ibutilide as an active control with intensive electrocardiographic sampling and signal averaging as a sensitive method to assess the effects of tadalafil on the human QT interval. J Am Coll Cardiol. 2005 ; 46: 678–687. CrossrefMedlineGoogle Scholar
- 63 Morganroth J, Ilson BE, Shaddinger BC, Dabiri GA, Patel BR, Boyle DA, Sethuraman VS, Montague TH. Evaluation of vardenafil and sildenafil on cardiac repolarization. Am J Cardiol. 2004 ; 93: 1378–1383. CrossrefMedlineGoogle Scholar
Uses of Viafill
Viafill is a drug belonging to the group of hormones, hormones. Manufactured in the form of film-coated tablets and packaged in boxes of 2 blisters x 2 tablets.
Viafill medicine has the main ingredient Tadalafil and other excipients, just enough for 1 tablet. Viafill is used to treat erectile dysfunction in men.
2. Dosage – How to take Viafill
2.1. How to take Viafill Drug Viafill is manufactured in the form of film-coated tablets, used orally, in tablets.
2.2. Dosage The dose of Viafill drug depends on each subject, the physiological progress of the patient will have an appropriate dose. Here is a reference dose of Viafill as follows:
Initial dose: For people using Viafill for the first time, the usual use is 10 mg of Tadalafil, need to take the drug before having sex. Depending on the tolerability and effectiveness of Viafill for each individual patient, the dose may be increased to 20 mg of Tadalafil or may be reduced to 5 mg of Tadalafil. The maximum dose of Viafill for patients is 1 time / day. Men who are experiencing erectile dysfunction need to be careful when using the appropriate dose of Viafill, because Tadalafil is effective up to 36 hours after taking the drug. For men with renal failure: For mild renal impairment, no dose adjustment is required. Men with moderate renal impairment should start using the drug at a dose of 5mg Tadalafil and a maximum dose of 10mg Tadalafil over a 48 hour period. For men with severe renal impairment, the maximum dose is 5 mg of Tadalafil. For men with liver failure: If men have mild and moderate liver failure, the dose should not exceed 10mg of Tadalafil / day. It is not recommended to use Viafill for men with severe liver failure, because it will be harmful to the patient’s health.
3. What to do in case of an overdose of Viafill?
Research results show that, for men with normal health, when using Viafil at a single dose of 500 mg of Tadalafil or a dose of 100 mg several times a day, there are unwanted side effects. In case of male abuse, drug overdose, it is necessary to notify a doctor or medical facility to apply symptomatic and supportive measures.
4. Contraindications to taking Viafill
Viafill should not be used in the following cases:
Do not use Viafill for patients who are being treated for other diseases with drugs containing organic nitrates in any form. Hypersensitivity: Do not use this Viafill for men who are hypersensitive to Tadalafil or any of its ingredients. Normally, people who are allergic to any of the ingredients in Viafill should not take the drug. Patients should carefully read the instructions for use and follow the instructions of the doctor before using the drug.
5. Viafill drug interactions
The following are some of the reported Viafill drug interactions such as:
Do not use Viafill with Cytochrome P450 inhibitors such as: Ketoconazole, Ritonavir, Erythromycin, Itraconazole because it will increase the concentration of Tadalafil in the drug. . Do not use concurrently with cytochrome P450 inducers such as: Rifampin, Carbamazepine, Phenytoin, Phenobarbital. Concomitant use will cause a decrease in the concentration of Tadalafil in the drug. In the process of using Viafill, men should be careful not to drink alcohol, tobacco, alcoholic or fermented beverages. Because those agents will change the ingredients in Viafill. You need to study the details in the leaflet and follow the instructions of your doctor or pharmacist for more details.
6. Side effects of Viafill
During the use of Viafill, patients may experience some unwanted side effects such as:
Headache. Digestive disorders . Nose bleed. Facial flushing, back pain, muscle pain, limb pain, angina. Lower blood pressure. Heart attack. Heart palpitations, palpitations. Usually, the side effects of the drug will gradually decrease and go away when the patient stops taking the drug.
When the above symptoms appear, the patient needs to inform the doctor about the unwanted effects that he or she encounters when using Viafill for timely treatment measures.
Especially if the patient discovers rare side effects that are not included in the instruction sheet. Inform your doctor or medical officer immediately if you suspect any side effects of Viafill 20mg.
7. Precautions when using Viafill
Before starting treatment with Viafill, it is necessary to carefully investigate the patient’s history of allergy to Tadalafil, other excipients of the drug and other medical history of the patient.
Patients with cardiovascular disease: It is necessary to consider for patients with cardiovascular disease who are treated for erectile dysfunction before using Viafill. Sexual activity will be accompanied by cardiovascular activity levels.
Patients with congenital retinopathy including retinitis pigmentosa: It is not recommended to use Viafill.
Men who have penile pain for more than 4 hours need to immediately notify the doctor for timely treatment to avoid future effects.
In the process of using Viafill in combination with other erectile dysfunction drugs, safety and effectiveness have not been evaluated.
Do not use Viafill for pregnant women and lactating women.
When using Viafill, it will not affect the process of driving vehicles or production machinery.
8. How to store Viafill?
Need to carefully read the instructions for storage of Viafill 20mg that have been written on the package and the drug instruction sheet.
Patients need to check the expiry date of the medicine. If the drug is not used, it should be collected and disposed of according to the instructions of the manufacturer or the person in charge of medicine.
Store Viafill 20mg at room temperature, avoid direct sunlight or high temperature, because it can convert the ingredients in the drug.